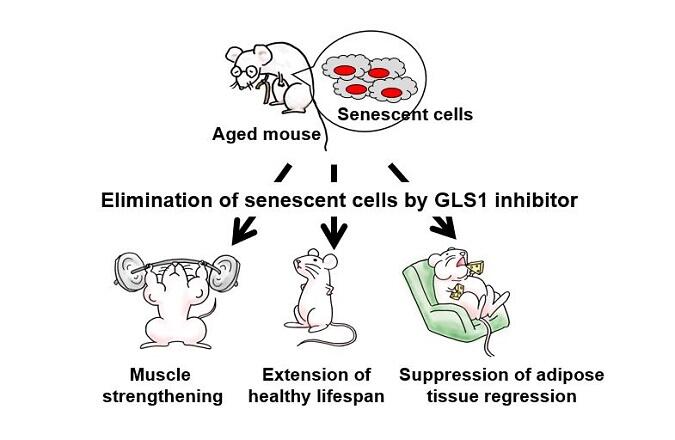
A research group led by Professor Makoto Nakanishi and Assistant Professor Yoshikazu Johmura at the Institute of Medical Science, the University of Tokyo has announced that they have succeeded in reversing the effects of ageing in mice. As glutaminase-1 (GLS1) is required for the survival of senescent cells, the cells were eliminated by the injection of GLS1 inhibitor. The study confirmed that injection of GLS1 inhibitor into mice carrying ageing-associated disease alleviated muscle-wasting, arterial sclerosis (hardening of arteries) and kidney damage related to ageing. The finding is expected to lead to the development of therapies to treat the broad spectrum of diseases that occur more frequently as we age. The findings were published in the January 15 edition of the international journal Science.
Apart from communicable diseases, ageing is a risk factor for most conditions from arterial sclerosis, high blood pressure and dementia to cancer. Organ tissue inflammation is regarded as a common cause of these diseases. Senescent cells accumulate with age. It was found that the non-inflammatory cells, which are not necessarily vital to the survival of the body, do trigger inflammation. It was also found that by removing the senescent cells using genetic engineering from ageing mice, geriatric pathologies such as arterial sclerosis and renal damage were alleviated. On the other hand, given the diversity of senescent cells, drugs that could eliminate them or a target are not yet known.
The research group has confirmed the accumulation of senescent cells in almost all organs as the body ages. Therefore, the group looked for the genes required for the survival of the senescent cells in order to develop drugs that could target and kill them exclusively. Specifically, senescent cells were infected with Lentiviral shRNA libraries, with comparisons made at Day 2 and Day 16.
They found that GLS1 was the essential enzyme in the glutamine metabolism of genes required for the survival of senescent cells. GLS1 induces senescent cell-specific gene expressions. GSL1 is an enzyme that changes glutamine to glutamate, producing glutathione. A secondary product of this process is ammonia, from the deamination. This is a process of many pathways, therefore partial blocking does not impact on the production of glutamate, but it does significantly restrict production of ammonia.
Further study of the senescent cells also revealed more damage to the lysosome membrane, which is the protease organelle (breaking down protein), than in healthy cells. Normally, this kind of damage to a cell reduces pH levels and leads to cell death, but in senescent cells the ammonia produced neutralizes the pH, allowing their survival. Moreover, damage to the lysosome means that cells can become proinflammatory, even if they do not become senescent.
It was found that injecting GLS1 inhibitor into normal and senescent cells not only limited the production of ammonia, it also led to the selective death of the senescent cells only.
When the inhibitor was injected into a 2-year-old mouse (equivalent to around 60 in human years), its rate of renal sclerosis, serum creatinine value and uric acid level all showed that it dramatically improved kidney function, which deteriorates with age. Improvements were also seen in pulmonary fibrosis and inflammatory cell infiltration of the liver.
Physiological function was also improved by the injection of the inhibitor. For example, muscle strength was improved in the ageing test mice. Serum cytokine levels dropped when they normally rise with age, so inflammation was clearly reduced. Diabetes challenge test values also improved in the diabetes test mice on high-fat diets.
Benefits were also seen in the fastest increasing side-effect of ageing, arterial sclerosis, as plaque coverage reduced in existing cases.
The research group has already confirmed that GLS1 manifests more with age in humans and mice alike. The GLS1 inhibitor had no effect on healthy cells, while it was confirmed that it had the same effect on cultured human cells. As in mice, if we are able to remove senescent cells that cause inflammation in humans, it is likely to lead to effective treatments for a wide range of diseases.
The group will now seek new GLS1 inhibitors as treatment candidates.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




