A research group that included Professor Shoen Kume, Associate Professor Nobuaki Shiraki and graduate student Shinpei Yoshida of the School of Life Science and Technology, Tokyo Institute of Technology, in a research collaboration with Professor Hiroyuki Kusuhara and Associate Professor Kazuya Maeda of the Graduate School of Pharmaceutical Sciences, University of Tokyo, and Kanto Chemical Co., Inc., researcher Teruhiko Watanabe, has succeeded in producing small intestine cells (iPS-intestinal cells) by using collagen vitrigel membrane to realize highly-efficient maturation of small intestine precursor cells derived from iPS cells.
Small intestine cells derived from human iPS cells offer promise as model cells for evaluating the absorbability of pharmaceuticals. This latest research outcome was achieved as a result of bringing together the research group's expertise in differentiation culture mediums, Kanto Chemical's collagen vitrigel membrane, and the pharmacokinetics analysis technologies of the Kusuhara Laboratory at the University of Tokyo.
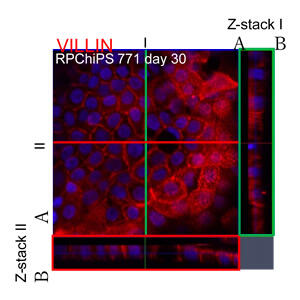
The research group produced small intestine cells that display villin positivity (villin is a marker molecule for small intestine cells), as a result of encouraging highly-efficient maturation of small intestine precursor cells through adhesive culturing on a collagen vitrigel membrane that offers excellent cellular adhesiveness and stretch activity. The small intestine cells displayed alkaline phosphatase activity, an indicator for maturation. Additionally, these small intestine precursor cells can be manufactured in large quantities from iPS cells, and be preserved by freezing.
When the iPS-intestinal cells that were produced were analyzed, it revealed that they were expressing the P-GP and BCRP genes, both drug transport molecules that are expressed in the small intestine, and the CYP3A4 gene, which is a drug-metabolizing enzyme. Furthermore, the results of function analysis showed that these drug transport molecules and the drug-metabolizing enzyme were in fact undertaking the transporting and metabolizing of substrates, respectively. Additionally, the iPS-intestinal cells were also found to be binding together strongly.
The results of an experiment in which the iPS-intestinal cells and drugs were incubated confirmed that with the passing of time the drugs were penetrating the iPS-intestinal cells, and that this permeability correlated closely with the oral absorbency of the drugs.
Shoen Kume explained, "Initially we had difficulty cultivating iPS-intestinal cells while maintaining barrier function, but by capitalizing on the experience accumulated thus far in developing methods to induce differentiation to the pancreas and the liver, we were able to construct a new cultivation method. As drug absorbability evaluation tool cells that display drug transport capacity and drug metabolizing capacity close to the small intestines of living things, these iPS-intestinal cells that we produced are expected to contribute to drug development as a result of being utilized for prediction research on the alimentary canal absorption rates of drugs, and on drug interactions in the drug metabolism and transport processes."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




