The development of all-solid-state batteries with solid electrolytes is being pursued worldwide, as the next-generation batteries of current alkali-ion batteries. A research group comprising Hiroshi Kageyama, Adjunct Principal Investigator (Professor, Graduate School of Engineering), Institute for Integrated Cell-Material Sciences(iCeMS), Kyoto University, and others succeeded for the first time in synthesizing antiperovskite compounds composed of soft anions, and revealed that the compounds display excellent properties as solid electrolytes for alkali-ion batteries. Lithium, hydrogen and sulfur were used for the compounds, but because various combinations are possible, the research has opened up a new horizon for antiperovskite compounds. Kageyama explains, "Once we ended up making the compounds it was simple, but the idea of using two varieties of anion had not been considered by ordinary researchers with a general knowledge of the field up to now, which is probably why no-one had done it." The results were published in Nature Communications.
Perovskite oxides are used in electronic devices as ferroelectrics, and progress is also being made with putting organic-inorganic hybrid perovskite compounds to use in solar cells. At the same time, compounds in which the roles of the cations and anions--the constituent elements of perovskite structures--have been reversed are known as antiperovskites, and Li3OCl, for example, holds promise as a solid electrolyte for all-solid-state batteries. However, because oxide ions and chloride ions have small polarizability, they end up having a strong coulomb interaction with the lithium ions, so there have been challenges to using Li3OCl as a solid electrolyte.

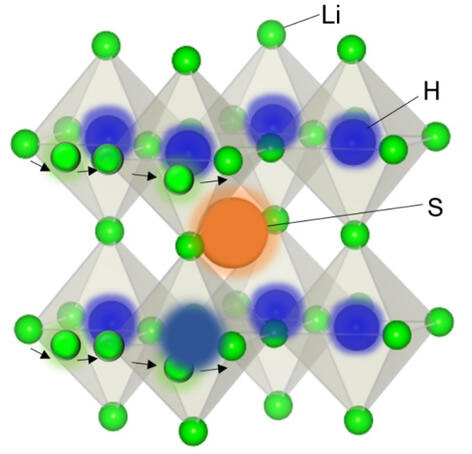
A research group comprising Professor Kageyama, Associate Professor Cedric Tassel and second-year doctoral course student Shenghan Gao of Kyoto University's Graduate School of Engineering; Program-Specific Assistant Professor Kentaro Yamamoto and Professor Yoshiharu Uchimoto of Kyoto University's Graduate School of Human and Environmental Studies; Japan Fine Ceramics Center Researcher Susumu Fujii and Senior Researcher Akihide Kuwabara; Assistant Professor Itaru Oikawa and Professor Hitoshi Takamura of Tohoku University's Graduate School of Engineering; and Assistant Professor Kotaro Fujii and Professor Masatomo Yashima of Tokyo Institute of Technology's School of Science, focused on negatively-charged hydrogen ions (hydride), whose properties include having one of the largest polarizabilities despite having only two electrons, and by combining them with chalcogen (sulfur which have a minus two valence, selenium and tellurium), which similarly has a large polarizability, successfully synthesized new substances, Li3HX and Na3HX, which have an antiperovskite structure.
Where these new substances are concerned, in the case of Li3HS, for example, it forms a structure in which octahedrons are formed by Li grouped around hydride at the center, and these octahedrons in turn surround the circumference of the S. An ideal environment is realized for an ion conductor, in other words, with an alkali metal ion existing within a soft (large polarizability) anion grid. In fact, the research group succeeded in demonstrating in theoretical calculations and experiments that the substances have extremely outstanding alkali ion conduction and extremely low ion diffusion activation energy.
In particular, because the substances have a soft anion grid, an ab-initio calculation showed that the octahedrons also rotate with extremely low energy, and this was also found to be supporting ion conduction.
The substances that were synthesized not only boast weak coulomb interaction and high ion conductivity as a result of using soft ions, but because the soft irons are also able to form good interfaces with negative-electrode active material, they could be described as ideal substances for solid electrolytes. Additionally, they do not use rare metals or other such materials and can be composed using only elements that exist widely on Earth. They can also be made at lighter weights, since they are 80% composed of light elements.
High pressure is necessary, but may be able to be synthesized in large quantities in future
Kageyama said, "Although high pressure is needed, because the substances can be synthesized extremely simply, we believe it will be possible to synthesize them in large quantities in the future. Because it is possible to synthesize a variety of types by changing the component materials, we believe they will be worked on worldwide. With ordinary solid electrolytes one component material promotes ion conduction, but with these substances a number of materials in the form of rotating octahedrons work together to enhance ion conductivity. This knowledge will also be useful in substance design going forward."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




