A research group that includes Professor Shigeki Kiyonaka of the Graduate School of Engineering, Nagoya University, along with doctoral program student Kento Ojima, Professor Itaru Hamachi of the Graduate School of Engineering, Kyoto University and others, has developed a fluorescent labeling-method for the glutamate receptors relating to memory and learning in the brain. By using small-molecule compounds the research group was able to attach fluorescent labels rapidly and without inhibiting receptor movement, which allowed it to quantitatively evaluate the movement of glutamate receptors. Kiyonaka says, "If there are selective ligands present it will be possible to apply the method to other neurotransmitter receptors. Additionally, because this method uses small-molecule compounds, there is a possibility it will also be able to be used in the diagnosis of diseases in the future." The research results were published in Nature Communications.
Information transfer within the brain takes place as a result of glutamic acid being released from synapses on one side, and receptors on the other side accepting the glutamic acid. When making memories or learning, the information transfer efficiency increases as a result of an increase in the expression level of AMPA receptors, a type of glutamate receptor. On the other hand, even though NMDA receptors are also a type of glutamate receptor, when cerebral strokes occur these NMDA receptors become abnormally active and trigger neuronal cell death, leading to brain dysfunctions.
Understanding the movement of AMPA receptors and NMDA receptors would not only clarify the mechanism of memory, it would also contribute to the diagnosis of neurological disorders. However, the labeling method that has been used up to now, which employs fluorescent proteins, has presented challenges. Namely, because the fluorescent proteins are large compared to the receptors, not only do they inhibit the intrinsic functions of the receptors, but they also end up making it possible to see even the receptors inside cells, which makes it impossible to distinguish which receptors are which.
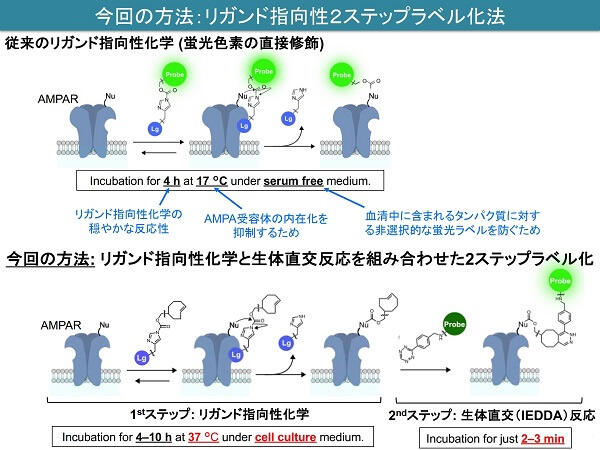
In 2017 the research group discovered the CAM2 small-molecule compound, which attaches fluorescent labels to AMPA receptors. As a result, it became clear that the movement of AMPA receptors differs to that observed with GFP. However, quantitative analysis of movement within cells was not possible because two to four hours were needed for the fluorescent labeling.
In the method that the research group has now developed, initially a small-molecule compound that is able to attach marks to AMPA receptors is sprinkled over the cells. Next, a fluorescent small-molecule compound that reacts rapidly and selectively to those marks is sprinkled over. Employing this approach has made it possible to carry out fluorescent labeling in two to three minutes.
When this method was actually used to analyze the movement of AMPA receptors, in model cells (HEK293T) derived from animals in which AMPA receptors were forcibly expressed it was found that when the AMPA receptors were expressed on the cell membrane they would be absorbed into the cells and for the most part end up breaking down in a few hours. Additionally, when the same analysis was carried out with nerve cells, it was found that with nerve cells the lifespan of the AMPA receptors was six times longer. The receptors were less likely to break down and remained even tens of hours later. Further analysis revealed that with nerve cells and model cells, the ratios of AMPA receptors that exist inside and outside cells do not change, but with nerve cells, although the AMPA receptors are recycled across a time period of several dozen minutes, with the model cells there is almost no recycling. Kiyonaka says that "AMPA receptors are being recycled at a good frequency, and conceivably this forms the molecular basis that governs memory."
In addition, as a result of designing and synthesizing a new compound, the research group succeeded in the rapid fluorescent labeling of NMDA receptors also. It was found that even with NMDA receptors, there is a receptor lifespan difference of around five times between model cells and nerve cells.
As a result of this newly-developed method, it has become possible to quantitatively evaluate the movement of AMPA receptors and NMDA receptors at arbitrary times, which is expected to contribute significantly to clarifying the molecular mechanism of memory. Furthermore, since this method does not employ genetic modification and so on, it makes it possible to directly study the intrinsic movement of receptors.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




