A research group led by Kohei Kusada, Program-Specific Associate Professor at the Hakubi Center for Advanced Research, Kyoto University and Professor Hiroshi Kitagawa and Program-Specific Assistant Professor Dongshuang Wu of the Graduate School of Science, Kyoto University, have succeeded in developing a water electrolysis catalyst that is 1,000 times more active and 10 times more stable than existing catalysts. The new catalyst structure has its highest atomic density where it touches the liquid. Kitagawa says, "At first it happened by accident, but through precise control of the atomic structure of the catalyst, we were able to design a highly stable catalyst. The effect of the metal structure on the activity and stability of catalysts has not been studied much to date. In future, we intend to analyse the detailed structure during the reaction to find out the origin of the high activity. We also hope to develop a good, low-cost, highly performing catalyst."
In the quest for a hydrogen-fueled future, hydrogen is being produced using a range of methods. The West, in particular, is pursuing electrochemical splitting (electrolysis) of water to produce hydrogen using renewable energy. However, a bottleneck for hydrogen production using electrolysis of water is the fact that the catalyst on the oxygen-generating side tends to dissolve due to oxidization.
In electrolysis of water, platinum and carbon are some of the most-commonly used catalysts for hydrogen evolution reactions (HER), while iridium oxide is often used to catalyze oxygen evolution reactions (OER). As an OER catalyst, ruthenium is the most active, but it is also the least stable. In terms of price, ruthenium (Ru) has the advantage that it is five to 16 times cheaper than iridium (Ir).
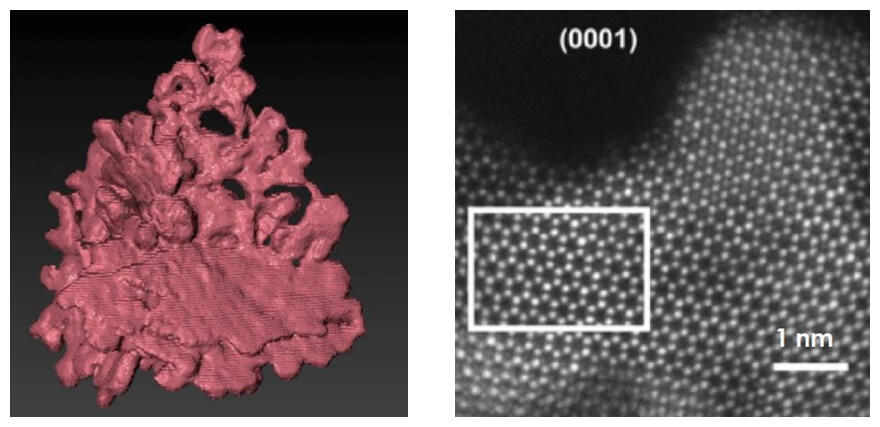
Right: The nanocoral crystalline structure of the anisotropic RuIr
(Credit: Kyoto University/Kohei Kusada)
The OER catalyst developed in this study is a combination of three nanometer-thick sheets (nanocoral) made up of 94% ruthenium and 6% iridium. The nanosheets have a hexagonal closed-packed crystalline lattice plane structure, exposing a hexagonal atomic arrangement on the top and bottom surfaces. In short, the surfaces reacting to the solution are close-packed crystal planes.
In a synchrotron X-ray powder diffractometry using an atomic resolution scanning transmission electron microscope and the SPring8 to investigate nanocoral OER catalyst activity, it demonstrated higher activity than previously reported catalysts including monometallic ruthenium, iridium nanoparticles and spherical Ir and Ru alloys. Activity was three magnitudes higher than typically used iridium oxide. In terms of stability, the RuIr alloy lost activity after an hour, and Ir nanoparticles lost activity after 12 hours, but the new catalyst was still electrolyzing after 122 hours at an unchanged level of activity.
Moreover, using the Ultra-High Voltage Electron Microscope at Kyushu University to investigate the behavior of nanosheets with the same structure as the nanocoral nanosheets during electrolysis, the researchers observed that while an ordinary nanosheet would break down into an amorphous state, the nanosheets exposing closely-packed crystalline surfaces was resistant to dissolution. They found that the key to a highly active, highly stable catalyst was a nanostructure designed with crystalline surfaces.
Furthermore, looking into its performance as an HER catalyst, it demonstrated more activity than single-metal nanoparticles or spherical RuIr nanoparticles and the same performance as a platinum catalyst. As such, it can also serve as an HER catalyst. In fact, in its use as both an HER and OER catalyst in electrolysis of water, it demonstrated better performance and stability than commercially available electrolysis cells, with the electrolysis continuing for 120 hours without loss of performance.
Nanocorals can be synthesized by a simple liquid phase reduction method, whereby a heated metallic salt solution containing Ru and Ir is sprayed into a mixture of triethylene glycol (reducing agent) and polyvinylpyrrolidone (protectant). FURUYA METAL Co., Ltd. is considering mass production, with samples currently being shipped to relevant organizations.
Many unknowns remain in the development of highly efficient OER catalysts in acid solution. Previously, there was not thought to be any effect on the performance or stability of a catalyst as a result of its crystalline structure. Looking ahead, the research group intends to find out more about the structure of catalysts during reactions and the reaction mechanism through in-situ measurement and theoretical research, with the hope that this will lead to the design of new catalysts.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




