The research group including Professor Shinji Toyota, Assistant Professor Eiji Tsurumaki, and Masters' student Kei Fujise of the Department of Chemistry, School of Science, Tokyo Institute of Technology has succeeded in synthesizing a novel long helicine molecule by extending the rings of a helical molecule, achieved by linking multiple benzene rings in a spiral.
(Credit: Tokyo Institute of Technology)
Helically structured molecules have distinctive optical properties due to their chiral structure and have been extensively used recently as a target molecule in organic synthesis. Helicenes, which are a polycyclic aromatic hydrocarbon, are the classic example of a helical molecule and known for their chiral optical characteristics.
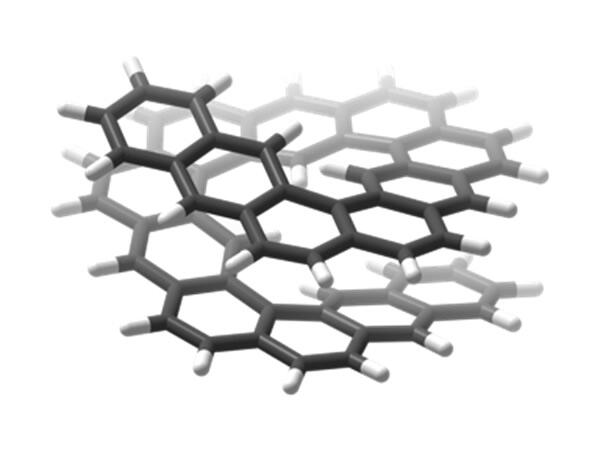
The substructure of anthracenes, an aromatic compound, is fused (when two benzene rings are bonded together) into a continuous V-shape, enabling the synthesis of a new helical molecule featuring large spirals. By inserting benzene rings regularly into the helicine, the research group constructed an extended helicene molecule containing spirals with an external diameter of over 1.2 nanometers, using a metal-catalyzed reaction.
An anthracene is a linear chain of three benzene rings. This new extended helicine is made of multiple anthracene fused in a spiral structure. These have been named "extended helical structures made of anthracene chains" ([n]HA: where n is the number of anthracenes fused together). So far, extended helical structures made of anthracene chains have involved up to five anthracenes.
In addition, the 3D structure of each molecule was analyzed using single-crystal X-ray diffraction and quantum chemistry calculation. The team found that where there were four or more anthracenes, they created at least one full turn in the spiral, and that most of the single and double anthracene molecules had a layered structure. Based on estimates, [5]HA molecules are unique helical structures displaying an average of around 1.5 turns, the most of any existing extended helicine molecules.
Furthermore, using spectrometry, as the helical structure extends from [2] HA to [5] HA, they found progressive changes in aspects such as light absorption and color tone. In particular, in [4]HA and [5]HA molecules with more than one turn, excited states were preserved longer as a result of interactions between layered anthracene rings, creating luminescence.
As Shinji Toyota explains, "by extending the helical structure, it should find applications as a molecular-sized spring, or 'molecular spring.' Moreover, extending the structure sideways may suggest a 'twisted graphene' character. We want to develop an aromatic compound that demonstrates circularly polarized luminescence, with a molecule able to divide enantiomers (mirror image isomers). Such chemical substances could be used as new elements in 3D displays or holograms, or applied to highly secure encryption."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




