A joint research group comprised of Associate Professor Takuya Tsujiguchi of the Faculty of Mechanical Engineering, Institute of Science and Engineering, Kanazawa University, Professor Yasufumi Takahashi of the Nano Life Science Institute, Kanazawa University, Assistant Professor Tatsuhiko Ohto of the Graduate School of Engineering Science, Osaka University, and Associate Professor Yoshikazu Ito of the Faculty of Pure and Applied Sciences, University of Tsukuba, has successfully achieved high efficiency formic acid synthesis by leveraging the surface action between redox graphene (rGO) and tin (Sn) in the synthesis process using electrochemical reduction, while also explaining the reaction mechanism.
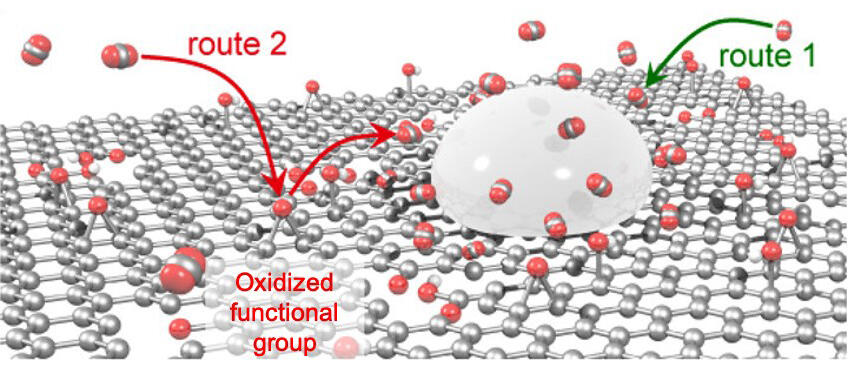
The process of synthesizing formic acid, an energy carrier, using CO2 and electricity from renewable energy is garnering attention as a technology for reusing CO2 emitted from factories and converting it to a resource. However, there still remains room to improve the efficiency of the formic acid synthesis process. Particularly, there is a need for a catalyst carrier (a material to serve as a carrier to hold the catalyst) that will enable the continuous and efficient supply of CO2 in the process.
Accordingly, the research group identified rGO as a carrier for efficiently absorbing CO2, and used it to carry an Sn catalyst, which is relatively easy to handle. When the Sn/rGO catalyst was compared to a pre-existing Sn catalyst, the introduction of the carrier achieved a four-fold increase in CO2 absorption by volume. Furthermore, the addition of rGO to Sn as a catalyst for formic acid achieved 1.8 times more efficient synthesis (Faraday synthesis).
The group was also able to observe the efficient reduction of CO2 on the Sn/rGO interface via scanning electro-chemical cell microscopy, making them the first team in the world to successfully visualize the synthesizing of large amounts of formic acid at the intersections between the catalyst and carrier due to the continuous supply of CO2 absorbed by the carrier being supplied to the catalyst.
Driven by a desire to help solve the environmental problem
According to Associate Professor Tsujiguchi, "These results explain how CO2 reduction proceeds efficiently through the strong CO2 absorption properties of the carrier, and this may eventually serve as a basic technology for synthesizing, via electromagnetic reduction, not only formic acid, but other substances that start a catalyst reaction by absorbing CO2 such as methanol, methane, and olefin. Accordingly, we hope to apply these results to the development of a high efficiency electromagnetic CO2 reduction device to help solve the environmental problem."
■Redox graphene: Graphene oxide is oxidized graphene that contains an oxygen function including a hydroxy group, a carboxy group, and an epoxy group. When subsequently reduced, the result is called reduced graphene oxide, or redox graphene. While graphene oxide is electrically insulated, redox graphene is electrically conductive, so it is expected to be useful as an electrode material.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




