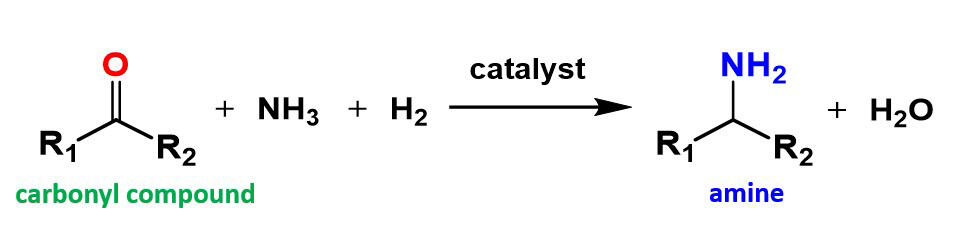
The research group of Associate Professor Takato Mitsudome of the Osaka University Graduate School of Engineering Science has succeeded in developing a nanocatalyst (phosphorated cobalt nanorods) that exhibits extremely high activity in reductive amination reactions for carbonyl compounds, which are important in the chemical industry.
In recent years, due to the depletion of rare resources and cost issues, there has been active research on non-precious metal catalysts as an alternative to precious metal catalysts. However, non-precious metal catalysts used in reduction reactions, such as reductive amination reactions, are still low in activity and require high pressure hydrogen. Moreover, because these catalysts are immediately oxidized and deactivated in the atmosphere, there is the risk of ignition and the catalysts themselves also have low durability.
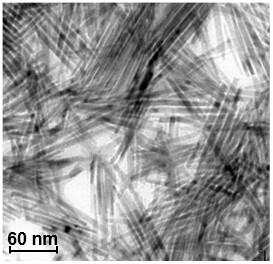
Credit: Osaka University.
In order to solve all of these problems, the research group worked on the development of a non-precious metal catalyst with high activity, durability, and safety. As such, they added phosphorus to cobalt, a cheap and available non-precious metal, and alloyed it into a nano-sized rod-shaped nano-alloy (Co2PNR), which was found to be stable in the atmosphere and highly active in reductive amination reactions for carbonyl compounds.
Additionally, Co2PNR shows the highest activity in the world amongst conventional non-precious metal catalysts and can convert a variety of carbonyl compounds to primary amines with high selectivity. This was the first time in the world that a non-precious metal catalyst was able to convert carbonyl compounds to primary amines under the extremely mild condition of hydrogen at normal atmospheric pressure (1 atm). Furthermore, because the catalyst that was developed is a solid catalyst, it can be easily analyzed by filtering it from the reaction solution, it can be repeatedly used, and it has become a 'smart catalyst' that combines safety, durability, and high activity.
Associate Professor Mitsudome said, "There are strong demands from the next-generation chemical industry, which aims to build a sustainable society, to develop safer, more energy efficient, and lower-cost catalytic reaction processes. Because the non-precious metal catalyst that we developed has high activity, durability, and safety, we want to demonstrate that the catalysts currently used by the chemical industry can be replaced with phosphorated metal catalysts in a variety of reactions, not just in reductive amination reactions."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




