Professor Kaneko's Group from Kyoto University discovers molecular mechanisms with clinical big data
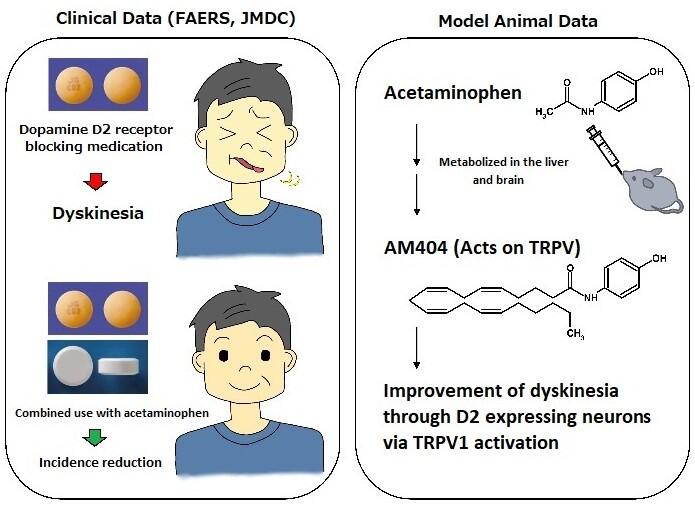
Drug repositioning will advance through analysis of the clinical data and reproduction of the results in animal experiments. Professor Shuji Kaneko's research group from Kyoto University Graduate School of Pharmaceutical Sciences PhD students, including Koki Nagaoka, analyzed clinical big data and discovered that the side effects of dyskinesia caused by long-term use of a schizophrenia drug was suppressed by the combined use of the antipyretic analgesic acetaminophen and identified its molecular mechanism. It is expected to lead to drug repositioning of acetaminophen as well as new drug discovery. This research was published in JCI Insight.
Tardive dyskinesia is a side effect caused by long-term use of a drug for treating schizophrenia that blocks the dopamine D2 receptor, and symptoms such as vacuous chewing movement and the inability to stop tongue protrusion even when it moves on its own can occur. However, its neural mechanisms are still unclear
.The research group has been working on finding new drug discovery targets from clinical evidence for about five years. Animal models of human diseases can be created and utilized for studying side effects of drugs. For example, gastric ulcer models using aspirin and indomethacin and diabetes models using streptozotocin have already been established. Therefore, we thought that if the side effects of a therapeutic drug used for a certain disease could be alleviated by a concomitant drug, it could be used as a prophylactic or therapeutic drug for treating the side effects and the biomolecules that cause the side effects could be identified.
A search conducted about the adverse event FAERS self-report including approximately 10 million cases published in the United States revealed that drugs that cause dyskinesia include dopamine D2 receptor blockers. The incidence of dyskinesia caused by aripiprazole was reduced in patients who were also taking acetaminophen, which is available as an over-the-counter cold medicine. Since FAERS does not describe the causal relationship between drug prescription and onset, a time series was analyzed using domestic insurance medical claim receipt data of 5.55 million cases of drugs sold by JMDC. From the results, it was revealed that the incidence of dyskinesia was reduced by 50% in 3 years with the use of acetaminophen compared to that without its use. Furthermore, the anti-dyskinesia effect of acetaminophen was reproduced in experimental animals.
When haloperidol (100 mg/kg body weight) was continuously administered to rats for 21 consecutive days, vacuous chewing movement (VCM) developed. However, the co-administration of haloperidol and acetaminophen significantly reduced the degree of VCM. The degree of VCM was significantly reduced even with a single oral dose of acetaminophen a day after continuous administration of haloperidol. Acetaminophen is mainly converted into two types of substances in the human body. In the liver, it is metabolized by the enzyme CYP2E to form a quinone body, which exerts an antipyretic effect in the brain via stimulation of the 'wasabi receptor' TRPA1. In the blood, it is hydrolyzed to para-aminophenol and associates with arachidonic acid to form a substance called AM404 in the brain. AM404 stimulates the capsaicin receptor TRPV1 in the brain to provide central sedation.
The investigation of the mechanism of anti-dyskinesia action clarified that when AM404 was administered to the brain, dose-dependent suppression of dyskinesia, as seen in acetaminophen, occurred. When a TRPV1-deficient situation was produced in mice and the degree of VCM caused by haloperidol was examined, the decrease in VCM caused by acetaminophen disappeared. In other words, the anti-dyskinesia effect of acetaminophen occurs via TRPV1.
Hyperactivity disorders such as dyskinesia are caused by a decrease in the activity of indirect tract neurons that control movement in the dorsal striatum in the brain. Therefore, immunostaining was performed with an antibody against c-Fos, a neural activity marker, and an antibody against preproeenkephalin, which can identify striatal indirect tract neurons, and the results were compared. The number of c-Fos-positive cells decreased in wild-type mice in which haloperidol caused VCM, and was recovered by acetaminophen, but no improvement in the symptoms was observed after administration of acetaminophen in TRPV1-deficient mice. In addition, a viral vector was introduced that can directly activate mouse striatal indirect tract neurons with N oxide (CNO). Haloperidol was administered daily starting 1 week later, and CNO was administered 4 weeks later, which led to a decrease in VCM and increase in the number of c-Fos-positive neurons showing that activation of striatal indirect tract neurons is required for anti-dyskinesia action.
Through the analysis of clinical big data, this study enabled the discovery of a drug that reduces specific side effects and revealed its mechanism of action and location. This method can be used to examine other side effects, but for that purpose, a method for analyzing clinical big data for research is required. In addition, this experiment and statistical data demonstrate that side effects are reduced or eliminated even with a single dose of acetaminophen, so it may be possible to expand its application as a prophylactic/therapeutic drug. However, Professor Kaneko stated, "Honestly, the administration of acetaminophen alone provided sufficient data, but it is a very cheap drug. Therefore, it will be difficult for companies to take the initiative to conduct clinical trials to expand their application because they cannot recover the cost. Unfortunately, there is currently no system or government support for conducting such drug repositioning in doctor-led clinical trials and expanding its application."

This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




