Novel nucleic acid drugs that target the transcription factor PRDM14, specifically expressed in breast and pancreatic cancers, were developed by a joint research group comprising Hiroaki Taniguchi, Specially Appointed Associate Professor, Institute of Medical Science, Keio University School of Medicine; Professor Fumitaka Nagamura, Institute of Medical Science, The University of Tokyo; Dr. Yohei Miyagi, Director, Kanagawa Cancer Center Clinical Research Institute; Dr. Kazunori Kataoka, Director, Nanomedical Innovation Center; and Visiting Professor Kozo Imai, Institute for Genetic Medicine, Hokkaido University. The drug comprises a chimeric siRNA that targets PRDM14 and a Y-shaped block co-polymer (YBC) that delivers the drug to its target location. Animal model studies have confirmed that the drug can achieve tumor growth inhibition, metastasis control, and prolongation of lifespan. A paper regarding this discovery was published online in the International Journal of Cancer.
Currently, phase I clinical trials including patients with triple-negative breast cancer are being conducted at the Cancer Institute Hospital in Ariake. PRDM14 is not expressed in normal breast tissue but is expressed in tumor-specific tissues in approximately 60% of breast cancers. Similarly, while it is largely unexpressed in normal pancreas tissue and acute pancreatitic tissue, PRDM14 is specifically expressed in slightly less than 40% of pancreatic cancers. The study group used the siDIRECT program to design PRDM14 gene-specific siRNA. In doing so, they computationally evaluated all human genes with five mismatched sequences and carefully examined whether genes important for off-targets were contained therein. These findings enabled them to synthesize a double-stranded RNA/DNA chimera (chimeric type siRNA).
Chimeric siRNA boasts gene-knockdown efficacy equivalent to that of siRNA but are more stable in blood and induce less innate immune responses. For the drug delivery system, the YBC developed by Director Kazunori Kataoka was adopted. The YBC contains a polyamino acid covalently bound to a branched polyethylene glycol chain. YBCs and siRNA are associated by their charges, forming a unit polyethylene complex (uPIC) that surrounds siRNAs. "The simple mixing of YBC and siRNA solutions allows one to easily prepare uPICs," said Director Kataoka. "This technology is quite easy to apply to the clinical setting because it does not need to be produced via complicated manufacturing methods. We have already constructed GMP -compliant systems (Good Manufacturing Process) that enable the mass production of YBCs." As uPICs are not recognized as foreign bodies, their retention in the blood is improved. Furthermore, because each particle is approximately 18 nanometers in diameter, they are not excreted by the kidneys. Thus, it is possible to deliver siRNA efficiently to tumor tissues through the EPR (enhanced permeability and retention) effect.
Further increases in tumor size, even after 1 month, were prevented upon transplanting PRDM14-positive triple-negative human breast cancer cell lines into mammary glands of immunocompromised mice and intravenously injecting uPIC after the tumor had exceeded a certain size. When uPIC was injected intravenously into breast and pancreatic cancer metastatic models, the number of metastatic lesions reduced, their size decreased, and the survival time increased. The researchers note that in a model of metastasis of pancreatic cancer to the liver, there were few metastatic sites. "After one month of treatment and one month of follow-up without treatment, we found that the uPIC group did not have larger tumor sizes," said Dr. Yaguchi. "As we have found that PRDM14 imparts traits of cancer stem cells to cancer cells (resistance to chemotherapy, tumorigenicity, and distant metastatic potential), we believe that loss of cancer stem cell traits with uPIC treatment leads to long-term drug efficacy."
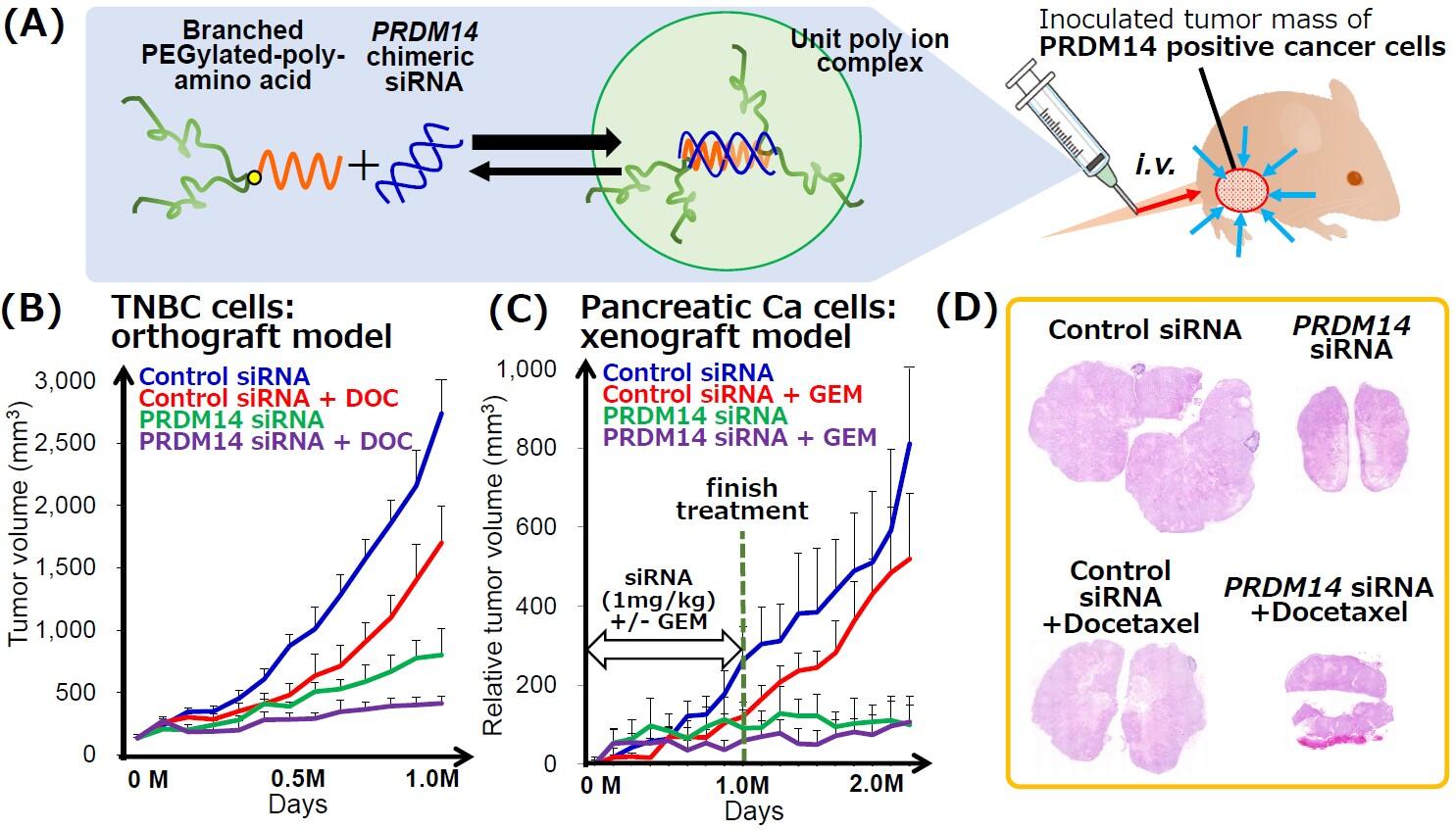
(B) Tumor volume of orthografttumors derived from PRDM14+triple negative breast cancer (TNBC) cells in mice administered uPICwith or without docetaxel treatment.
(C) Tumor volume of xenograft tumors derived from PRDM14+pancreatic cancer cells in mice administered uPICwith or without GEM treatment.
(D) Representative images of H&E-stained orthografttumors derived from PRDM14+ TNBC cells on the end point, related to figure B.
© 2021 Union for International Cancer Control
In an effort to accelerate the practical application of uPICs, the researchers and Nanocarrier Corp. began a physician-initiated phase I trial in women with triple-negative breast cancer in September 2020, and the company said there have been no major adverse events to date. They are also developing markers to noninvasively diagnose PRDM14 expression. These tests are being developed so that uPICs can enter the clinical market alongside companion diagnostics by the end of the Phase III trials. Associate Professor Taniguchi said, "When we begin our post-Phase II clinical trials, I would like a pharmaceutical company to join us."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




