A research group led by Assistant Professor Hideaki Morishita (currently a lecturer at the Graduate School of Medicine, Juntendo University), Specially Appointed Assistant Professor Tomoya Eguchi, Professor Noboru Mizushima, and colleagues at the Graduate School of Medicine of the University of Tokyo discovered.an intracellular degradation mechanism that enables transparency of the eye lens during eye maturation. Using live zebrafish, they successfully captured the images of organelle degradation in the eye lens. The phospholipid-degrading "PLAAT family" enzymes present in the cytosol were found to be responsible for this degradation process. They found that this system is also present in mice and is essential for acquiring transparency of the eye lens. These findings, published in the April 14th issue of the British scientific journal Nature, are expected to lead to an understanding of the differentiation mechanism of the lens cells and the intracellular degradation system.
The eye lens must be transparent for the eye to function properly, as light passing through the cornea and the thick lens is refracted, forming an image on the retina. The lens is composed of epithelial cells, as well as fibrous cells that differentiate from them, and all organelles in the cells disappear during the process of maturation, the mechanism behind which remained unknown.
In 2006, the research group found that autophagy, which is responsible for organelle degradation in normal cells, is not involved in organelle degradation in the eye lens.
This research group aimed to understand the mechanism underlying organelle degradation in the eye lens using zebrafish, in which genes can be easily modified and live cells are easy to observe.
First, using live zebrafish expressing green fluorescence inside the endoplasmic reticulum and red fluorescence inside the mitochondria, they monitored the increase in transparency of the eye lens every 5 min starting 60 h after fertilization. This allowed them to observe the degradation of organelles from the center to the outside of the lens as well as the diffusion of their contents to the surrounding cytosol.
Next, the research group searched for genes more strongly expressed in the lens than in other organs to understand the mechanism underlying this degradation process, which resulted in the identification of approximately 60 genes. They disrupted each of these genes in zebrafish using the CRISPR/Cas9 system and examined the occurrence of organelle degradation in each of the zebrafish mutants.
Organelle degradation did not occur in the lens of zebrafish lacking the Plaat1 gene. This gene was found to be conserved among vertebrates, from fish to humans, and encode an enzyme (phospholipase) that degrades various phospholipids.
The research group also showed that the mitochondria and endoplasmic reticulum are degraded 50-70 h after fertilization in the lens of normal zebrafish, whereas the degradation does not occur in Plaat1 gene-deficient zebrafish. Under an electron microscope, the center of the normal lens was filled with crystallin proteins 96 h after fertilization, whereas organelles remained in Plaat1 gene-deficient zebrafish. In addition, the enzyme encoded by the Plaat1 gene appeared to be required for the degradation of organelle membranes. Next, they examined how the enzyme attacks the membranes of organelles; results showed that the enzyme, usually present in the cytosol, migrated to the organelle membranes immediately before the degradation of organelles. The enzyme appeared to function by piercing the membranes with the hydrophobic region present in the enzyme, resulting in the degradation of the organelle.
Mice have three enzymes similar to the one encoded by the Plaat1 gene, of which Plaat3 is highly expressed in the lens. When examining the function of this enzyme in mice, the research group found that the organelles in the center of the lens of normal mice were degraded half a day after birth, whereas the organelle degradation did not occur in Plaat3 gene-deficient mice. The lens of Plaat1 gene-deficient zebrafish exhibited white turbidity and refractive error 6 months after fertilization, when the lens of normal zebrafish is transparent.. In addition, a similar abnormality was observed in Plaat3 gene-deficient mice 9 months after birth, when the lens of a normal mouse is transparent.
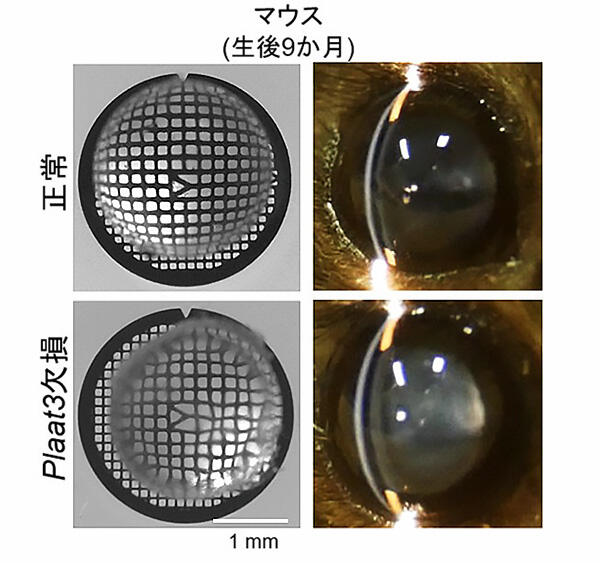
A. Slit-lamp examination of the lens of 9-month-old mice. Plaat3-deficient mice demonstrate cataracts (left).
B. Bright-field images of a grid pattern through the lens from 9-month-old mice. The grid pattern is distorted through the lens of Plaat3-deficient mice, indicating refractive defects (right).
Credit: Assistant Professor Hideaki Morishita
The degradation of organelles, such as the nuclei and mitochondria, that occurs during lens maturation has been known for hundreds of years, but this study discovered its underlying mechanism for the first time.
The research group found that these enzymes are also expressed in other organs and that organelle degradation does not occur even if they are forcibly expressed in cells of other organs. In organelle degradation in the lens, slight damage to the organelle membranes is observed prior to degradation, which indicates that the enzyme selectively carries out this degradation process. In addition, nuclear DNA degradation occurs almost normally even when the gene encoding the enzyme was deleted, suggesting that this degradation process is carried out by the DNase leaked from the lysosome.
It has been suggested that Hsf4, a transcription factor located upstream of the Plaat1 gene, is involved in the degradation process. In addition, as this enzyme degrades only membranes, other degradation processes are likely regulated by different mechanisms, and the research group aims to understand the relationship of such mechanisms in the future.
In addition, cataracts, which develop with general aging, are caused by protein denaturation, and they do not involve the mechanism discovered in this study.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




