The collaborative research group of Professor Emeritus Hajime Tanaka of the University of Tokyo, Associate Professor Peng Tan of Fudan University, China, and Associate Professor Xu Limei of Peking University, China, clarified the conditions and mechanisms for the realization of low-temperature high-speed crystallization, based on real-time observation and numerical simulation at the single-particle level of the crystallization process in a colloidal dispersion system.
Normally, crystallization proceeds by the transport of molecules via diffusion (called denitration) During denitration cloudiness occurs in optical fibers which would normally be transparent. For example, medications manufactured in glassy states that are well absorbed by the human body can crystallize during storage. This results in the medications being poorly absorbed, leading to serious ramifications. However, it has not been explained why crystallization occurs at low temperatures at which such molecules are hardly able to move.
The research group followed the dynamic process of crystallization at single-particle resolution. A dispersion system of colloidal particles was used at the micrometer scale. This is a 10,000-time scale-up of an atom size of ~1 Å. Thus, the group was able to clarify the entire process of low-temperature crystallization.
Numerical simulations revealed the following factors necessary for fast crystallization to occur at low temperatures:
- The existence of an uneven and thick crystal growth interface with a crystal precursor structure.
- The ability to repair disordered defects confined in the crystal after its growth.
The key factor that enables ultra-low-temperature crystal growth is the inherently mechanical instability of the precursor structure of the crystal growth interface. This mechanical instability drives the crystallization process. Through this investigation the research group was able to discover a new mechanism by which crystallization proceeds in a manner similar to the domino effect driven by mechanical instability, even in the absence of diffusion. Professor Emeritus Tanaka said, "The clarification of the physical mechanism of vitrification (denitration) is expected to enable not only the stabilization of the glass state (inhibition of crystallization), but also the formation of high-quality crystals, which is expected to contribute significantly to a variety of industrial applications."
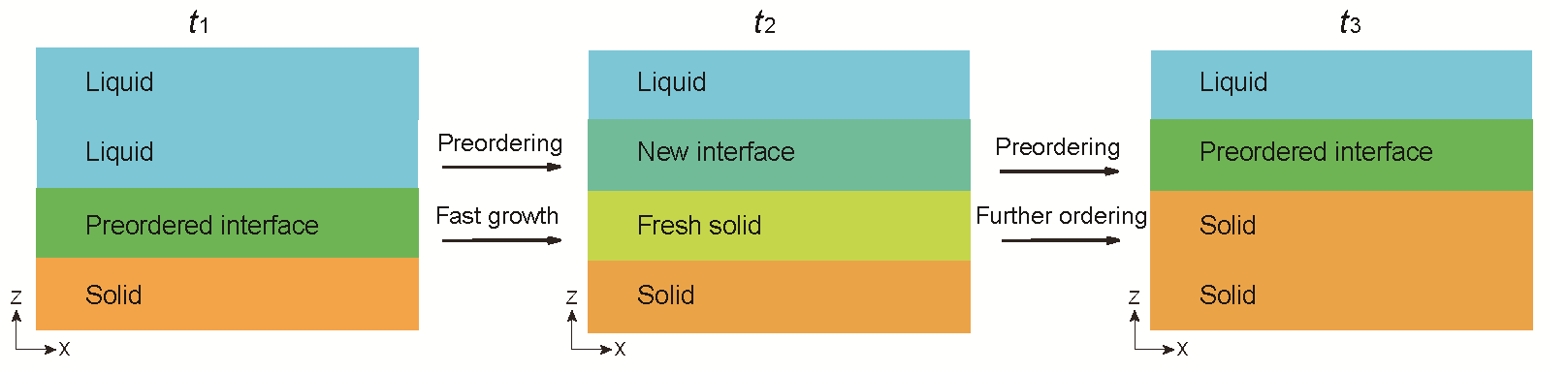
Credit: Professor Tanaka, the University of Tokyo
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




