Inhalation of low concentrations (i.e., 5-40 ppm) of nitric oxide gas causes only the pulmonary blood vessels to dilate and so enhances oxygen uptake capacity. Therefore, it is used to treat hypoxic respiratory failure with pulmonary hypertension (i.e., prolonged pulmonary hypertension) in newborns and alleviate pulmonary hypertension in cardiac surgery. In addition, nitric oxide has anti-inflammatory, antithrombotic, and antiviral properties. However, nitric oxide is toxic at high concentrations, reacting with atmospheric nitrogen to produce highly toxic nitrogen dioxide. Therefore, it must be handled with care and is typically stored in heavy and bulky high-pressure cylinders.
Although small and inexpensive nitric oxide generators are being developed worldwide, controlling their chemical reactions requires many high-precision instruments. The initial investment cost and need for regular maintenance are both issues that must be resolved. Shinsuke Ishihara (Senior Researcher) and Nobuo Iyi (Research Fellow) of the International Center for Materials Nanoarchitectonics (MANA) Frontier Molecular Group at NIMS have developed a disposable nitric oxide generator that can supply nitric oxide gas at a stable concentration for longer than half a day. Nitric oxide can be generated at clinically suitable concentrations by using moist air to induce the ferrous iron-assisted reduction of nitrogen dioxide. These researchers harnessed this process to develop inexpensive and compact nitric oxide generators that can be used in settings outside of hospitals and in developing countries. The full research has been published online as a Forum Article in a special issue of Inorganic Chemistry titled "Renaissance in NO Chemistry."
In 2020, the research team reported that when a mixture of nitrite ion-containing layered double hydroxide (LDH) and iron sulfate powder is packed in a plastic column and exposed to a flow of humid air, nitrogen monoxide is generated easily and safely via the exchange of nitrite ions between the LDH layers and the sulfate ions. The released nitrite ions react with divalent iron ions, resulting in the reduction of the nitrogen dioxide. However, the mass synthesis of LDH, which contains nitrite ions, and the short generation time of nitric oxide presented practical challenges. By adding a heat treatment process to the LDH synthesis process, mass production was realized using inexpensive commercial raw materials and water. In addition, structural defects generated during the heat treatment process moderately suppress the reaction, which stabilizes the nitric oxide generation process. The mixture of nitrite ion containing LDH and iron sulfate can be stored safely while being introduced into the column. Upon introducing a humid air flow, the reduction of the nitrite ions by divalent iron ions proceeds gradually, yielding nitric oxide. The amount of nitric oxide generated, and the rate of generation, can be controlled by altering the composition and amount of the initial mixture: only 2 g of the solid mixture produces a clinically acceptable concentration of nitric oxide (40 ppm, 250 ml/min) for longer than half a day. Therefore, a continuous supply nitric oxide can be maintained by simply replenishing the solid mixture twice a day.
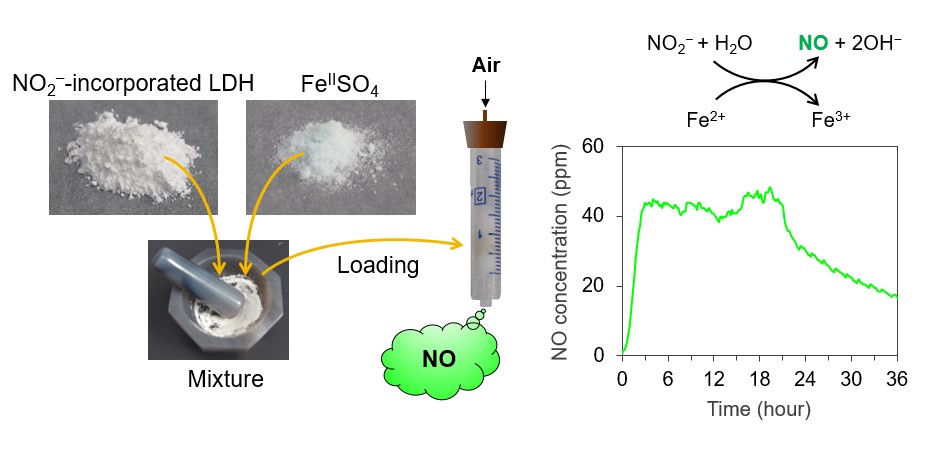
(Adapted with permission from Inorg. Chem.
(DOI: 10.1021/acs.inorgchem.1c00456). Copyright (2021) American Chemical Society.)
Respiratory failure, cases of which are currently increasing worldwide owing to COVID-19, poses a significant threat to life. Significantly, the proposed nitric oxide generator can be used anytime and anywhere and shows great potential for treating neonatal pulmonary hypertension in developing countries as well as preventing the aggravation of sudden respiratory failure due to infectious diseases, and other causes.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




