A research group consisting of Associate Professor Kazusaku Kamiya, Assistant Professor Ichiro Fukunaga, and their colleagues at the Juntendo University Graduate School of Medicine, Department of Otorhinolaryngology, announced on May 18 that they had successfully reproduced the most common pathology of hereditary hearing loss in inner ear cells generated from patient iPS cells. They generated inner ear cell gap junction-forming cells from patient iPS cells and confirmed a marked reduction in gap junction material transport, believed to be the cause of this type of hearing loss. The results will be useful for drug screening and therapy development for patients and were published in the international scientific journal Human Molecular Genetics.
Hearing loss is the greatest risk factor for dementia and attempts to treat dementia by treating underlying hearing loss have received worldwide attention. Hereditary hearing loss is a frequent disorder that affects approximately 1 in 1600 live births and while medical devices, such as hearing aids and cochlear implants, have been invented, no drugs exist for the condition. To date, approximately 140 causative genes have been identified for hereditary hearing loss. However, GJB2-mutant hearing loss accounts for nearly half of all patients with the condition. Connexin (CX) 26, produced by the GJB2 gene, is a component of the gap junction that transports ions between cells in the inner ear. By preserving the ionic composition of the inner ear lymph, ions flow into the gap junction and vibration is converted into neural activity, resulting in the ability to hear. Therefore, GJB2 mutations prevent gap junctions from maintaining their structure, resulting in hearing loss.
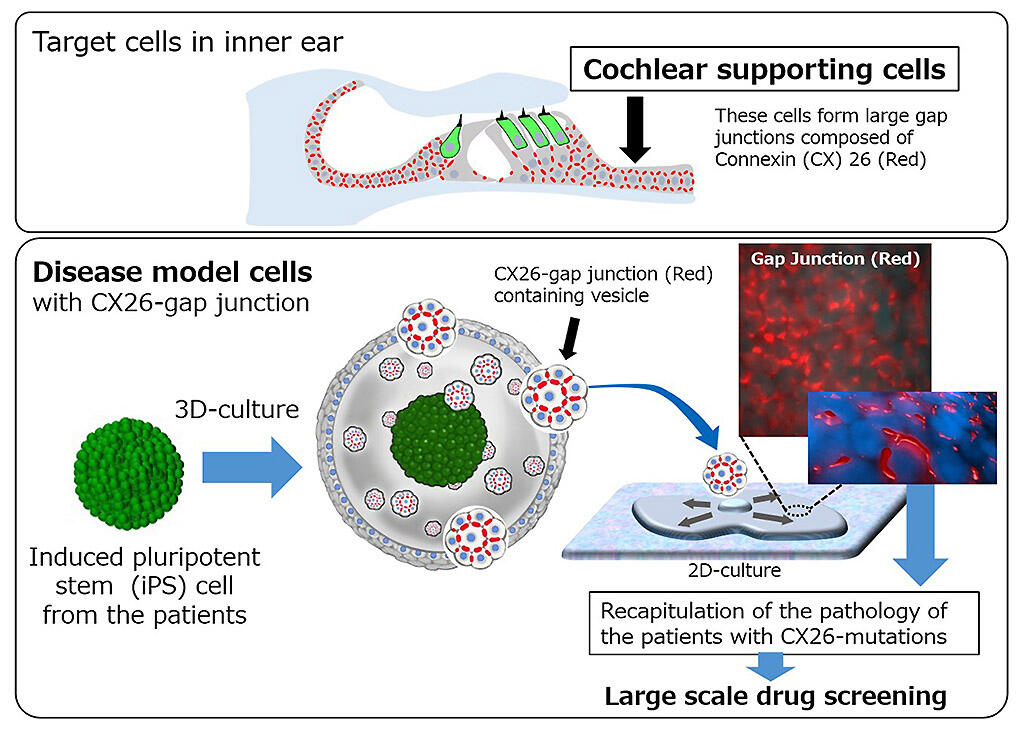
Previously, this research group has advanced gene therapy and drug development for this hearing disorder and clarified the disease state mechanism. Among these endeavors, to screen for potential drugs, the group produced a cell sheet made from a group of inner ear cell groups from the iPS cell of a deaf mouse, which lacked the GJB2 gene in 2016, and succeeded in reproducing the disease state. However, reproducing the disease state in human iPS cells has not yet been realized. The study group received blood donations from two individuals with typical profound hearing loss due to GJB2 variants (with cochlear implants) and created iPS cells. By refining the method for generating cell sheets established in mouse iPS cells, they created a group of inner ear supporting cells (cochlear supporting cells) forming a network of gap junctions in human iPS cells. More specifically, culture using the efficient three-dimensional aggregation method was performed to search for conditions that highly expressed CX26 and caused the differentiation of human iPS cells into ectoderm. They found that the addition of insulin markedly increased CX26 expression.
Next, the cells forming CX26 gap junctions contained in the culture mass were separated and propagated. Cells were cultured on the uniquely developed cochlear cells. Consequently, cell sheets forming gap junctions by CX26 were created. Gap cells in the created cell sheets were smaller in size than those in healthy individuals, and decreased function was confirmed from a substance permeation test and so it was confirmed that the disease state was reproduced.
Credit: Juntendo University
Using the previously prepared cell sheets, the group performed screening using the compound library, and the candidate compound was clarified multiple times. In the future, the AMED project aims to explore compounds as potential therapeutic agents for further treatment and pioneer gene-editing therapies using adenovirus-associated vectors that have already been developed. The group aims to clarify the mechanism of severity by comparing patient mutations and symptoms. They hope that this will lead to different options for inner ear treatment. "If you can select a therapeutic drug by screening, you may be able to respond to lifestyle modification and food additives if the disease is mild," Professor Kamiya said. "We expect various uses that can be used in regenerative medicine for the inner ear and safety and efficacy evaluation of drugs for the inner ear in the future."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




