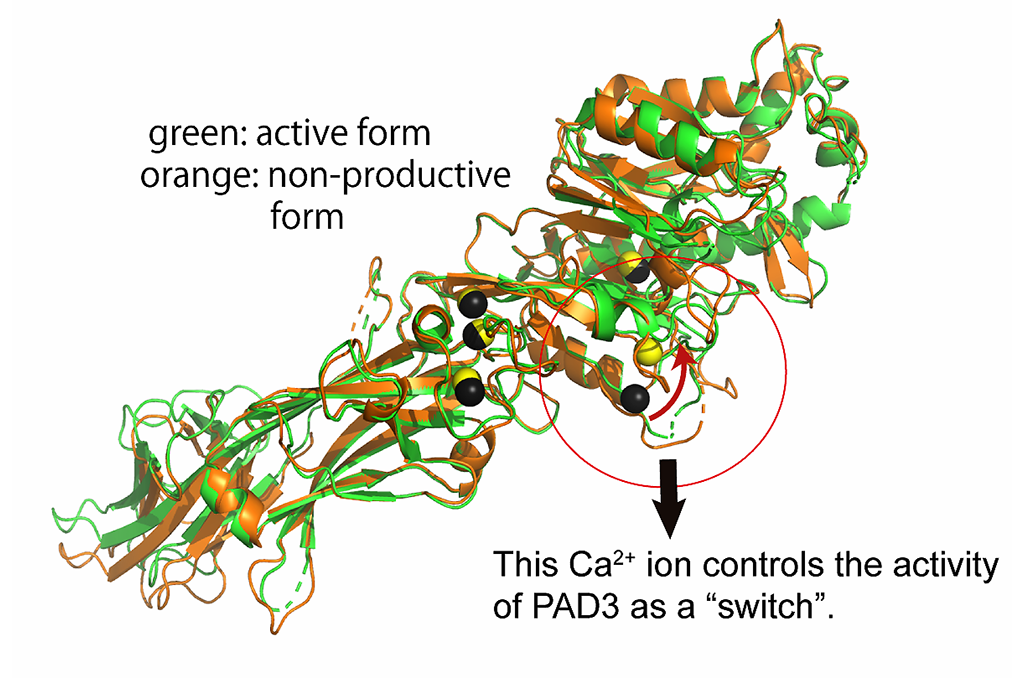
Professor Masaki Unno and colleagues at the Graduate School of Science and Engineering, Ibaraki University, successfully determined the detailed structure and properties of the enzyme PAD3, which is involved in the formation of the "cuticle" (the outermost layer of the hair), which controls hair luster and tension. PAD3 is one of the enzymes (protein arginine deiminases) that convert arginine to citrulline* in targeted proteins in response to elevated intracellular calcium levels. PAD3 mutation is also known to be a factor in "uncombable hair syndrome." Its substrate specificity differs from that of isozymes PAD1 and PAD2, which are also expressed in hair follicles. Three-dimensional structural information of PAD3 is needed to determine the difference in substrate specificity and for the development of PAD isozyme-selective inhibitors. The research team expressed human PAD3 genes in Escherichia coli and obtained large quantities of human PAD3, the protein of interest. The obtained PAD3 was then purified to obtain monocrystals. These obtained crystals were subjected to X-ray crystallographic analysis at the large synchrotron facility Spring-8, and six conformations of PAD3, including active and inactive forms, were clarified.
Credit: Ibaraki University
It was found that the structure and properties of PAD3 closely resembled those of PAD4, which is not expressed in hair, rather than those of PAD1 and PAD2, which are expressed in hair. The researchers also found that PAD3 undergoes a structural change when bound to calcium, completing the arrangement of amino acids involved in activity of the enzyme. In addition, the structure of PAD3 bound to an inhibitor that suppresses enzyme activity and its structure in the inactive state were confirmed and were compared with those of a similar enzyme. These analyses revealed structural features (spaces) unique to PAD3 at the compound binding site, suggesting the possibility of developing a PAD3-selective agent by utilizing these spaces. Professor Unno said, "In the future, we wish to clarify the mechanisms underlying hair cuticle formation by designing structure-based isoenzyme-selective inhibitors and conducting structural analysis of complexes with S100A3, one of the substrates of PAD3, and citrullinated and structurally altered S100A3".
■ Citrulline is an amino acid produced by the conversion of arginine and is not encoded by genes.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




