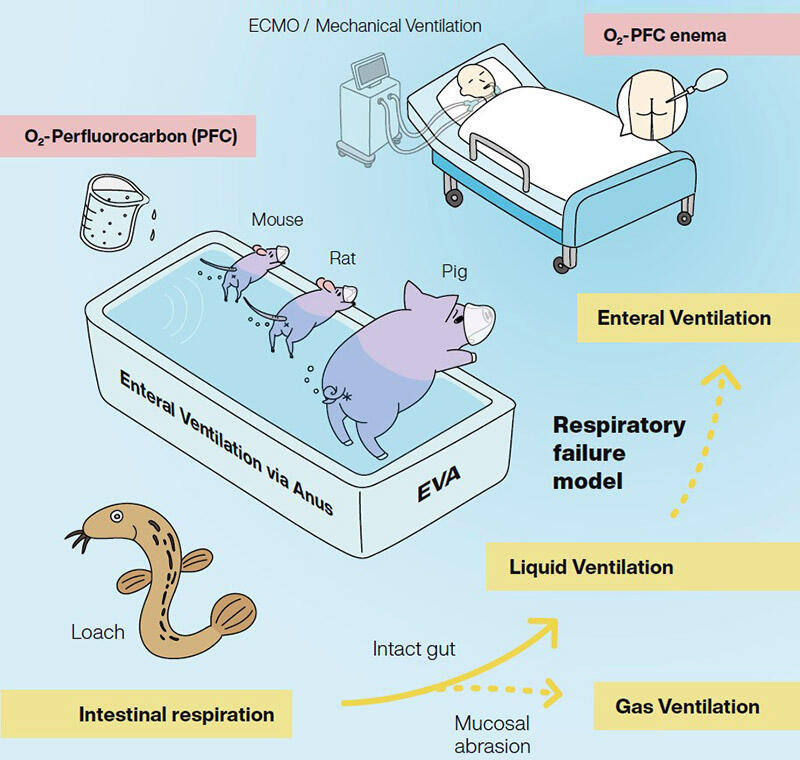
A research group consisting of Professor Takanori Takebe and Part-Time Lecturer Ryo Okabe from the Tokyo Medical and Dental University Integrated Research Organization, in collaboration with Nagoya University Graduate School and Kyoto University, announced on the 15th of May that they developed an enteral ventilation (EVA) method that can deliver oxygen to the blood of the entire body by delivering oxygen to the intestine.
The application of the mechanism of intestinal respiration in the marine loach, which lives in a hypoxic environment, can be tested in mammals. The research group tested if the application of the EVA method could be expected to improve respiratory failure in model animals. The results are expected to lead to development of a new circulatory/respiratory management method for respiratory insufficiency.
For respiratory insufficiency with low blood oxygen levels, which frequently occurs in serious respiratory illnesses such as pneumonia and COVID-19, circulatory respiratory care is provided using a ventilator and an artificial lung (extra-corporeal membrane oxygenation, ECMO). One issue with this method is that the ventilator exerts pressure on the lung during oxygenation, resulting in lung strain. On top of this, during ECMO, the occurrence of blood clots and other complications, in addition to excessive costs, are problematic. A simple and minimally invasive cardiorespiratory management method is required to solve these problems. Therefore, the research group developed an EVA method to increase the blood oxygen concentration via gas exchange through the intestine, adapting the mechanism present in the loach.
The loach is known to inhabit areas such as paddy fields and swamps. In these hypoxic environments, it performs intestinal respiration, taking up oxygen from the intestine. Intestinal respiration in the loach is facilitated by the presence of multiple capillaries surrounding the intestine, with abundant blood circulation. In a hypoxic environment, the digestive and absorptive functions are weakened, and the epithelial intestinal mucosa becomes thin. However, the feasibility of this mechanism was yet to be investigated in mammals. Therefore, oxygen was administered into the intestine of mice through the anus and survival experiments were performed under a hypoxic environment.
In the non-treated group, the survival rate was 0% at 5-6 minutes. However, in the treated group, vital functions could be maintained for approximately 10 minutes Particularly, in a group in which the epithelial intestinal mucosa was removed to simulate a condition like that in the loach, functions were maintained for approximately 1 hour. Arterial oxygen tension also improved in the group in which the epithelial intestinal mucosa was removed. Next, the utilization of a perfluorocarbon (PFC) with high oxygen dissolution was examined as epithelial intestinal mucosa removal may lead to a high risk of hemorrhage in the patient. PFCs are compounds composed solely of carbon and fluorine, which are immiscible with water and blood, and are biologically inactive. Oxygen dissolves well in these substances. These compounds have been used as ophthalmic surgery materials and lung lavage fluids, with verified safety.
Designed by Asuka Kodaka
In this study, researchers compared the oxygenation achieved with intestinal administration of 1 mL of oxygen-containing PFC1 (administered via an enema-like procedure) with that in a no-treatment condition, using a hypoxic chamber to maintain the atmospheric oxygen concentration at approximately 10%. The no-treatment group remained stationary without performing any activity in the chamber; however, the treatment group continued to move vigorously. When continuously measured for approximately 1 hour in each group, the oxygen saturation continued to decrease in the no-treatment group. However, in the treatment group, the oxygen saturation decreased once immediately after initiation, but recovered after 10 minutes and was maintained until 1 hour. Oxygen concentrations exhibited similar patterns and no serious adverse events were reported in safety studies in rats.
In addition, with the cooperation of Dr. Hideki Kobayashi, a professor at Keio University School of Medicine, the authors conducted an experiment by administering multiple doses of 400 milliliters of PFC with dissolved oxygen under similar hypoxic conditions. The results confirmed that oxygen saturation, partial pressure, and concentration were improved by the treatment. Both mice and pigs were expected to recover as needed. The researchers observed oxygen being slowly absorbed from the administered PFC through the intestine.
In the future, further efficacy and safety evaluations will be conducted, followed by clinical trials after 1-2 years. First, these researchers are aiming to achieve approval for the application of this technique in a medical device to assist in switching from ventilators and similar devices. Subsequently, they hope to achieve approval of this technique as a drug, allowing the treatment to be used during first aid for aspiration asphyxia. As the safety of PFCs has already been verified, its approval as a medical device is expected to be relatively quick.
Professor Takebe said, "In the past, regardless of COVID-19, there were only options such as ECMO for respiratory failure; however, if we can apply a method that uses PFC, we will have more options or support. In that sense, I think it might be beneficial to patients. I will do my best to deliver it to patients as soon as possible."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




