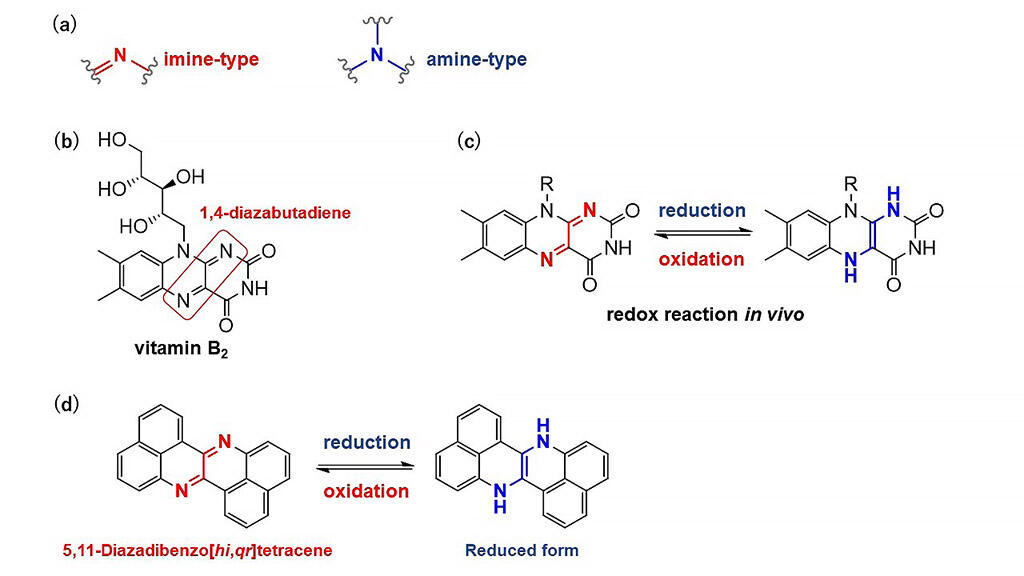
A research group led by Assistant Professor Keisuke Fujimoto of Shizuoka University, Faculty of Engineering, has succeeded in developing a new organic dye that exhibits redox activity. Polycyclic heteroaromatic (PHA) compounds are a promising class of molecular compounds as candidates for functional organic materials, and exhibit unique functions depending on the characteristics of the heteroatoms they are made up of. Among heteroatoms, imine-type nitrogen atoms exhibit a high degree of oxidation, while amine-type nitrogen atoms exhibit a low degree of oxidation. Although the heteroatom is same for both cases, these groups exhibit contrasting functions. Due to these functions, PHA compounds, which can undergo mutual conversion between both groups through a redox reaction, are a potential class of novel organic materials. The function of which depends on the switching between the two contrasting properties.
Regarding the basis of the study, Assistant Professor Fujimoto explained, "We planned to create a conceptually new, original molecule, with the belief that it would lead to the development of groundbreaking molecular functions. Inspired by porphyrin chemistry, on which I worked when I was a student, we designed a molecule focusing on the nitrogen atom." The research group designed and synthesized a new PHA compound (5,11-diazadibenzo [hi, qr] tetracene) exhibiting redox activity by incorporating the "1,4-diazabutadiene structure," which is the key structure responsible for the redox reaction of vitamin B2 in the human body and evaluated its properties.
This compound was found to be a red pigment with orange emission and high electron acceptability. Moreover, the mutual conversion to the reduced structure proceeded quantitatively and reversibly. Furthermore, it was clarified that chemical modification using an oxidative hydride substitution reaction was possible, and that the spectral characteristics could be easily controlled. Assistant Professor Fujimoto concluded, "While we succeeded in obtaining the original molecular skeleton, we are still exploring the areas to which it can be applied to. In the future, this material is expected to be applied as an organic semiconductor and be utilized in imaging technologies that are based on changes in emission color arising from redox reactions. Considering the redox reaction as a charge/discharge process, or absorption/desorption of hydrogen molecules, we believe that these materials can be utilized for the development of energy technologies."
Credit: Shizuoka University
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




