A joint research group discovered that the distribution of exosomal shapes in body fluids may be a novel indicator for cancer diagnosis. The research team comprised Assistant Professor Sou Ryuzaki and Professor Kaoru Tamada, Institute for Materials Chemistry and Engineering, Kyushu University; Associate Professor Takao Yasui and Professor Yoshinobu Baba, Graduate School of Engineering, Nagoya University; Associate Professor Makusu Tsutsui, Professor Masateru Taniguchi, and Invited Professor Tomoji Kawai, Institute of Scientific and Industrial Research, Osaka University; and Professor Takahiro Ochiya, Institute for Medical Science, Tokyo Medical University. The results of the study were published in Analytical Chemistry.
"Our results were obtained by working with leading researchers in various disciplines, including physics and medicine," Dr. Ryuzaki said, adding that "we will continue to work on establishing multidisciplinary test technologies that can easily detect cancer." Exosomes (extracellular vesicles) are derived from cells and exist as lipid bilayer membrane-enclosed vesicles of up to 100 nm in diameter. They are present in body fluids such as blood and urine. Recent studies have revealed that exosomes are involved in cell-cell communication. Due to this characteristic, they have attracted attention as biomarkers for cancer cells, as they carry information regarding the cells from which they originate. In particular, the development of biosensors attuned to the miRNA present in exosomes has facilitated successful detection of several cancers. Physical features, such as shape and hardness, are also being increasingly recognized and studied.
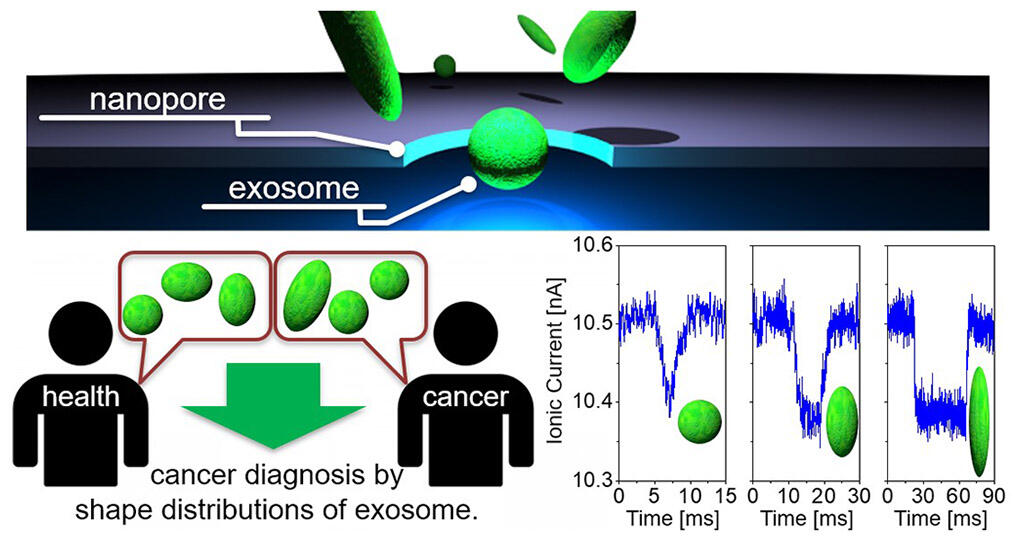
Although various shapes have been revealed using electron microscopy, several physical features of the exosomes remain poorly understood. This is because it is technically difficult to measure the shape and hardness of exosomes dispersed in solution (such as body fluids). This group of researchers successfully measured the shape distribution of exosomes derived from a variety of cells, including liver cancer cells, breast cancer cells, colon cancer cells, and mammary gland cells, using a nanopore device based on one-particle shape analysis technology, which was developed by them. The researchers discovered that the distribution of exosomal shapes differs among cell types. As the particles pass through the devices' 200 nm diameter pores, their cross-sectional volume is continuously measured based on changes in ion current. For example, exosomes derived from liver cancer cells were shown to comprise a mixture of spherical and ovoid (shaped like a rugby ball) particles, whereas exosomes derived from breast cancer cells were spherical. Furthermore, comparison of exosomes in the blood samples of a breast cancer patient and a healthy subject revealed different shape distributions. This difference allowed the group to differentiate between the breast cancer patient and the healthy subject. Although the shape distributions of other exosome types remain to be determined, this study indicates that the differential distributions of exosome shapes in body fluids can help to detect and identify different types of cancers.
Credit: Kyushu University
To evaluate the accuracy of this biosensor method, the researchers intend to measure the shape distribution of various types of exosomes present in the blood. They have set a target of 3 years to establish the practical test system. At the laboratory level, the duration from measurement to analysis is approximately 30 minutes for this technique. The researchers aim to reduce this duration to approximately 10 minutes. They expect that this comparatively simple technique will reduce cancer testing time significantly compared to testing miRNA present in exosomes.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




