Professor Tomoki Todo of the Center for Advanced Medical Research, Institute of Medical Science, The University of Tokyo, and the Japan Society for Gene and Cell Therapy announced on June 11 that G47Δ, a third-generation herpesvirus for cancer treatment, which was jointly developed by Professor Todo and Daiichi Sankyo, was approved as a regenerative medicine product for malignant glioblastoma. The drug is being marketed as Delytact (generic name: teserpaturev) and it is the first approved virotherapy product for brain tumors in the world. The therapeutic effects of the drug on all solid cancers have been confirmed, and its application is expected to expand in the future.
Studies on G47Δ, which was developed by Professor Todo and his research group, were consistently conducted under the initiative of academia, from the invention to practical application, and all clinical developments were conducted at the University of Tokyo. A five-year clinical trial, which commenced in 2009, was conducted at the University of Tokyo to verify the safety of the drug on patients with glioblastomas. Furthermore, from 2015 to 2020, a physician-initiated phase II clinical trial was conducted, and the drug's safety and efficacy were confirmed.
The malignant gliomas (grades 3 and 4) targeted by the drug are the most aggressive form of the cancer, which account for one-fourth of primary brain tumors. Glioblastomas are among the most common of tumors. They are also associated with a poor prognosis and are highly prone to recurrence. Even when undergoing surgery, radiation therapy, and chemotherapy, patients with the disease have a life expectancy of 18 months after diagnosis and a 5-year survival rate of 10%.
By modifying the viral gene, cancer virotherapy infects and destroys cancer cells using viruses that can grow only in cancer cells. "G47Δ" was prepared by modifying three genes of herpes simplex virus type 1. A major feature is that the immune system eliminates proliferated "G47Δ" alongside destroyed cancer cells; the cancer cells are recognized as foreign entities by the immune system, and anti-cancer immunity is induced. It has been confirmed that a therapeutic effect can be expected not only for tumors near the administration site but also for distant tumors. It has also been confirmed that cancer stem cells, which prevent the radical cure of cancers, are efficiently destroyed by the therapy.
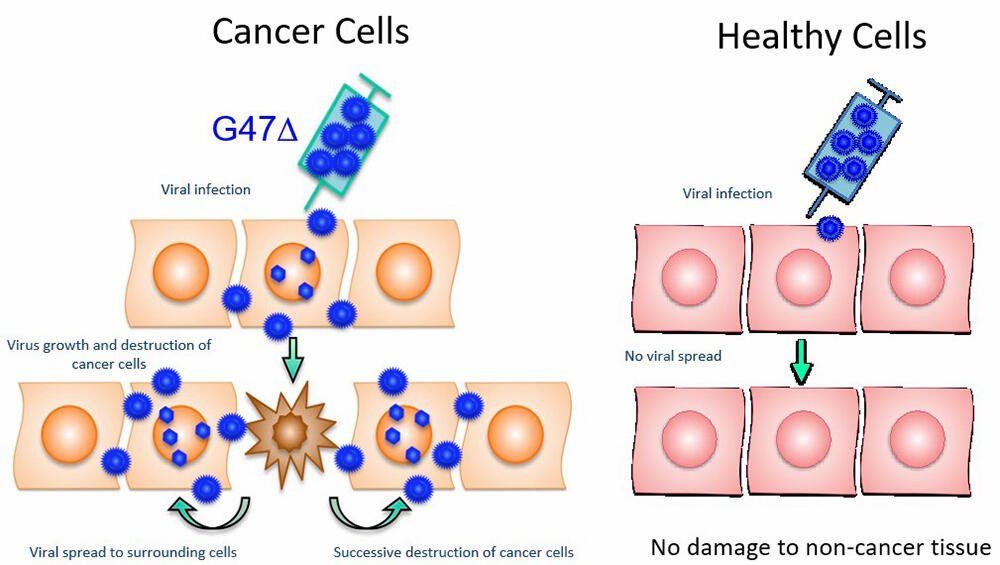
Credit: Institute of Medical Science, The University of Tokyo (with modifications)
In a phase II clinical trial, patients with recurrent glioblastoma were administered G47Δ virotherapy in addition to standard therapy. In patients receiving standard therapy alone, the life expectancy after recurrence of the disease ranged from 3 to 9 months; however, 5 out of 13 patients receiving both therapies survived more than 1 year, 3 patients survived more than 3 years, and 1 patient is still alive today. Clinical trials using this process have also been conducted for prostate cancer and olfactory neuroblastoma since 2013. The members of the study have stated that they will continue to work on expanding the application of this drug.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




