A variety of G protein-coupled receptors (GPCRs), which are membrane proteins, are responsible for different regulatory functions in the living body. As a result of their abundance, one third of drugs approved by the Food and Drug Administration (FDA) target GPCRs. Although GPCRs had been thought to exert certain actions in response to their agonists, it has been shown that their response changes depending on the mode of agonist binding.
In collaboration with Associate Professor Asuka Inoue of the Graduate School of Pharmaceutical Sciences at Tohoku University, Professor Eriko Nango of the Institute of Multidisciplinary Research for Advanced Materials at Tohoku University, and Engineer Kunio Hirata at RIKEN, the research group made up of Professor Masatoshi Hagiwara, Professor (and RIKEN Group Director) So Iwata, PhD student Shintaro Maeda, and others of the Graduate School of Medicine at Kyoto University used X-ray crystallography to reveal the three-dimensional structure of the receptor S1PR3--one of the GPCRs that recognize the lipid sphingosine monophosphate (S1P)--in the state of being bound to S1P. S1P is a lipid that, via its receptors, acts to control the transport of immune cells in the body, vascular permeability, and the development of blood vessels. Therefore, some therapeutic agents for inflammatory diseases, such as autoimmune diseases, specifically target S1P receptors. However, the mechanism through which S1P activates its receptors to transmit information to cells was not clearly understood. In this study, the research group found that S1P activates its receptors by straightening its lipid chain within the receptor. They also revealed a part of the mechanism by which the information transmitted to the cell is biased depending on the length of the lipid chain. These findings were published in Science Advances.
As explained by Professor Hagiwara, "Since the structure has finally been solved in this study, pharmaceutical companies around the world will make various compounds based on it. Also, the fact that the action of a receptor differs depending on the binding mode of its agonist will have great implications for drug discovery in the future." In the living body, lipids are known to be not only energy sources and components of cell membranes but also molecules that execute physiological activity via their receptors (lipid mediators). S1P, which is one of these lipid mediators, carries out various physiological functions via the GPCR S1PR1-5, mainly in the vascular and immune systems. Compounds targeting S1P receptors for the treatment of inflammatory diseases, such as autoimmune diseases, have been developed and marketed as therapeutic agents in recent years.
The research group found that a newly identified anticancer compound acted as an agonist of S1P receptors and that its transmitted signal was different from that of the endogenous agonist S1P. However, it remained unclear how S1P receptors change the signal transmitted into the cell depending on the type of agonist that was bound. The only known structure of S1P receptors had been that of agonist-bound inactive S1PR1, and even the activation mechanism of S1P receptors had not been identified.
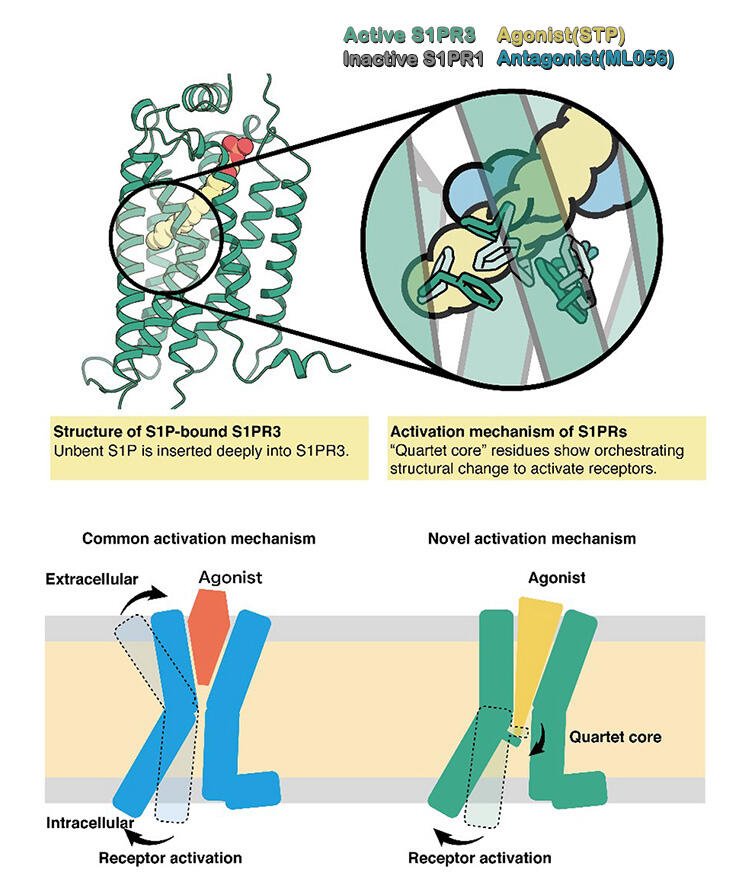
In this study, the research group determined the structure of S1P-bound S1PR3 by X-ray crystallography. They successfully collected crystals of S1PR3 suitable for obtaining high-quality data, which they achieved by crystallizing the S1PR3 protein with an antibody fragment that binds specifically to it, which had been generated by Professor Iwata's research group. The X-ray diffraction experiment conducted to obtain the data was performed at SPring 8, which is a synchrotron radiation facility. Data was automatically acquired from 316 crystals, which allowed the researchers to determine the structure. Whereas the S1P-mimicking antagonist bound to the inactive S1PR1 has an L-shaped bend, as seen in its structure revealed by a previous study, this study found that S1P penetrated its receptor obliquely with its lipid chain.
Mutation of the amino acid residues comprising the quartet core, which undergoes a structural change due to the oblique penetration by the lipid chain, weakened the receptor's activation in response to S1P, suggesting that this difference is strongly involved in receptor activation. Previously determined structures of GPCRs had led to the proposal that the binding of an agonist to the GPCR pocket facing outside the cell, which contracts the pocket, leads to receptor activation. However, the structure of S1P-bound S1PR3 revealed its novel activation mechanism as occurring through the agonist pressing the switch (quartet core) located deep in the pocket.
In addition to S1P, other receptors for lipids, such as prostaglandins and cannabinoids, have been structurally determined in their active form. However, they do not exhibit the binding mode of agonists that penetrate their receptors obliquely, as discovered in this study. These results suggest that S1P and its receptors may have uniquely acquired the activation mechanism of oblique penetration by an agonist.
Credit: Kyoto University
Additionally, on the basis of the binding mode of S1P, the research group hypothesized that shortening the lipid chain would change the active state of the receptor; therefore, they measured the receptor activity using S1P with 16 carbons. Having a shorter lipid chain than S1P with 18 carbons, which makes up the majority of S1P chains in the body, this S1P analog showed weaker activation than S1P with 18 carbons only in some signaling processes. Furthermore, analysis of the mutant revealed that the group of hydrophobic amino acid residues surrounding the lipid chain was involved in this phenomenon. These results indicated that a slight change in the mode of interaction between the lipid chain and the surrounding amino acid residues could induce a bias in the signal transduction controlled by the receptor.
In recent years, structure-based drug design (SBDD), which is the designing of drugs on the basis of structural information, has been enthusiastically carried out. The results of this study are expected to be the foundation of SBDD for diseases related to the S1P receptors.
This study revealed the structure of the S1P receptor that regulates the functions of the immune and vascular systems, showing that S1P activates its receptor by penetrating it obliquely. Additionally, the properties required for agonists to induce a bias in signal transduction have been revealed. However, the detailed mechanism of how an agonist with a shorter lipid chain induces a bias in signal transduction remains unclear. The research group aims to clarify this mechanism in the future by determining the structure of a receptor bound to its agonist with a short lipid chain. As explained by PhD student Maeda, "We aim to understand the signal transduction mechanism in more detail by analyzing the structure of S1PR3 in different states, as well as the structures of other receptors, in the future."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




