The research group comprising Yoshiya Seto (Associate Professor), Michio Kuruma (graduate student), and Taiki Suzuki (graduate student) of the School of Agriculture, Meiji University has announced its discovery that tryptophan can be used to control germination in broomrape (Orobanche minor), a root parasitic plant that causes considerable agricultural damage in Africa. This discovery was made by analyzing the effects of different tryptophan derivatives on germination in broomrape and it is anticipated that these findings will contribute to developing an effective broomrape control strategy. These results have been published in the US scientific journal Bioorganic & Medicinal Chemistry Letters.
Broomrape is a root parasitic plant native to the Mediterranean region and has spread worldwide through seed contamination of exported grains. Although established as an exotic species in Japan, broomrape does not grow well in the local nitrogen-rich, well-hydrated soils, and no major damage associated with its growth has been reported until date. Broomrape suppresses growth of plants in families such as Asteraceae, Leguminosae, and Umbelliferae by parasitizing their roots, causing damage to these crop families in arid regions worldwide. Root parasitic plants belonging to the genus Striga cause damage similar to that caused by broomrape.
As a control measure for these root parasitic plants, a suicidal germination control method that involves forced induction of germination and death has been promoted as a promising approach. The efficacy of root parasitic plant control was confirmed to be high when fields were sprayed with the plant hormone strigolactone (SL) prior to planting crops. This is based on the ability of these plants to sense SL, which triggers premature germination. However, owing to high synthesis costs, the application of SL to controlling broomrape may not be feasible. The research group, while screening for microorganisms that produce SL-like substances, examined whether components of the growth media used affect germination in broomrape. They found that tryptophan, which is widely used as a nitrogen source in soil media, inhibits the germination induced by SL in broomrape. To investigate further, compounds with chemical structures similar to that of tryptophan were administered to root parasitic plants to investigate their effects on germination.
Among the candidate tryptophan-like compounds, 10 commercially available compounds with side chains at the same positions as those in tryptophan and with indole rings, were selected. The researchers found that auxin (IAA), for which tryptophan is a biosynthetic intermediate, has a particularly strong germination inhibitory activity and can also inhibit seedling root elongation. Based on these findings, IAA-SL with a substructure of SL in IAA was synthesized and examined for its effects on broomrape germination. IAA-SL treatment was found to inhibit seedling post-radical growth after germination, while maintaining the level of germination-inducing activity of SL. It was also observed that N-acetyltryptophan, in which the amino group of tryptophan is acetylated, has a weak germination-stimulating activity.
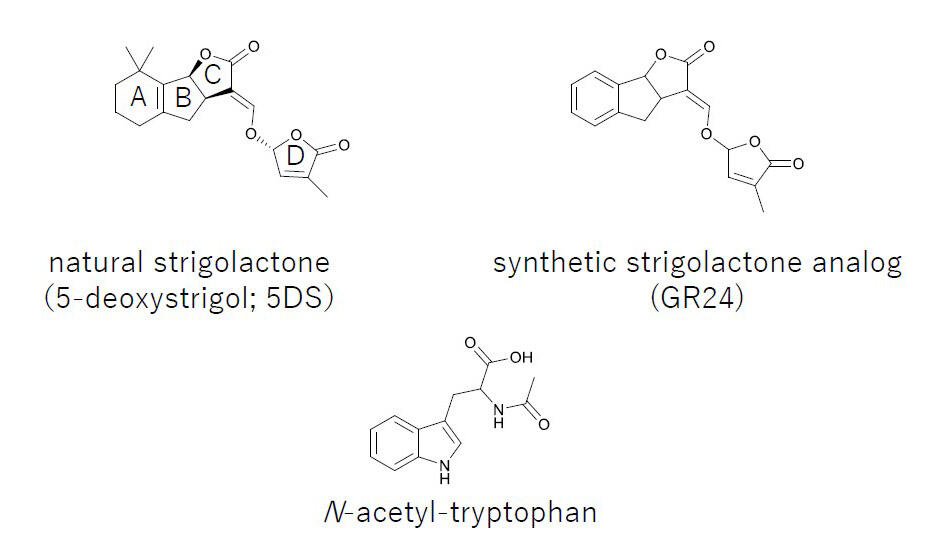
a synthetic strigolactone analog (GR24), and N-acetyl tryptophan (discovered in this study)
Credit: Meiji University
Subsequently, the researchers sought to determine whether this germination-inducing activity could be strengthened by examining the effects of different derivatives in which different substituents (CH3, OH, OCH3, NO2, Cl, and Br) were introduced into the indole ring. They found that these substituents had varying effects on the strength of the induction activity of the respective derivatives. Notably, these derivatives were between 10- and 100-fold more active than the unmodified N-acetyltryptophan. The derivative obtained by introducing a methoxy group (OCH3) as a substituent was found to be the most active. Although less active than SL, N-acetyltryptophan appears to be more useful if it is more potent than SL, given its relative ease of synthesis. Associate Professor Seto explains that, "As the compounds examined until date show relatively weak activity, I intend to synthesize molecules with similar structures but stronger activity. In order to achieve this, receptor identification is important. SL receptors have already been identified in Striga, and we will investigate whether N-acetyltryptophan acts on these receptors. I plan to design molecules with stronger activity by first performing docking simulations using receptor models."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




