In collaboration with the Deputy Chief of Technology Terumi Dohi, from the Sector of Fukushima Research and Development at the Japan Atomic Energy Agency, and Group Leader Yoshihito Ohmura, from the Division of Fungi and Algae at the Department of Botany at the National Museum of Nature and Science, researcher Hiroya Suno and Deputy Director Masahiko Machida, from the Center for Computational Science & e-Systems at the Japan Atomic Energy Agency, hypothesized that some of the metabolites synthesized by lichens are involved in the retention of radioactive cesium. By performing quantum chemical calculations, they successfully evaluated the ability of five metabolites produced by lichens found in Fukushima prefecture (e.g., Parmotrema tinctorum and Flavoparmelia caperata) to form complexes with cesium.
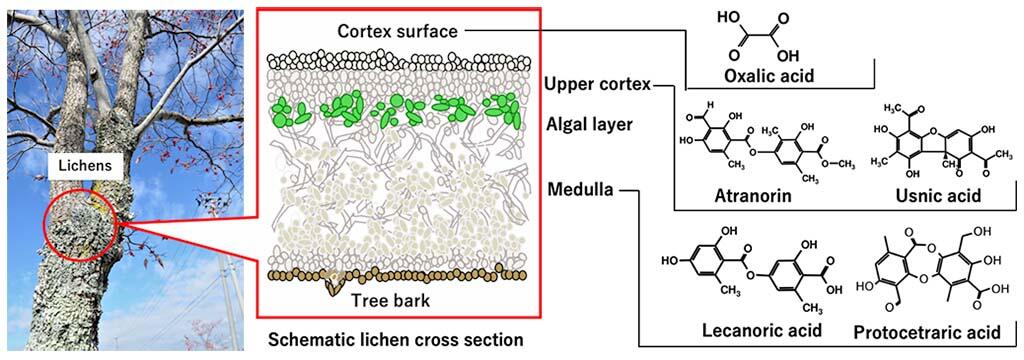
Lichens are a complex lifeform that is a symbiotic partnership of fungi and algae. They grow slowly in relatively firm or non-moveable areas, such as rock surfaces, tree trunks, houses, and concrete. Lichens reportedly retain radioactive cesium for long periods of time; however, clarifying the retention mechanism has been challenging to biologists as well as environmental chemists.
This research study was initiated after the Fukushima Daiichi Nuclear Power Station accident in 2011. Immediately after the accident, questions such as "Why does radioactive cesium strongly adsorb to clay minerals in soil?" and "Why do some mushrooms concentrate radioactive cesium?" were raised in close succession. Deputy Director Machida said, "Lichens secrete distinctive metabolites, which may play a role in the retention of heavy metals, but nothing is known about the retention of radioactive cesium."
In response to these issues, researchers focused on the widely observed lichen species Parmotrema tinctorum and Flavoparmelia caperata, which had been reported to retain radioactive cesium in Fukushima, and used quantum chemical calculations to evaluate the ability of five abundant metabolites (oxalic acid, atranorin, usnic acid, lecanoric acid, and protocetraric acid) to form complexes with cesium and alkali metals.
Credit: Japan Atomic Energy Agency (JAEA)
In the trees where these lichens grow, atranorin and usnic acid, which are produced in the typically alkaline upper cortex of lichens, exhibited strong ability to form complexes with alkali metals under alkaline conditions. Meanwhile, lecanoric acid and protocetraric acid, which are produced in the medullary layer of lichens, exhibited strong ability to form complexes with alkali metals under the conditions of neutral pH, with a low concentration of alkali metals.
These findings suggest that metabolites produced by different layers of lichens can form complexes with alkali metals, including cesium, appropriately depending on environmental conditions, and at least some lichens can retain alkali metal complexes in their different structures (e.g., upper cortex and medullary layer).
Deputy Director Machida said, "Production of unique metabolites by lichens allows them to inhabit a wide range of environments. For example, lichens produce UV-absorbing metabolites that allow them to prevent UV damage induced by strong direct sunlight. I think that many such metabolites will be useful for human life, and I hope to clarify the antiviral functions of certain metabolites, which could contribute to the development of new and effective antiviral drugs."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




