Genetic diseases are thought to be associated with the loss or over-strengthening of the function of a protein encoded by a particular gene. In collaboration with NIH, Rockefeller University, Chiba University, Hiroshima University, and Kazusa DNA Research Institute, the research team of Professor Tomohiro Morio and Assistant Professor Motoi Yamashita of the Graduate School of Medical and Dental Sciences at Tokyo Medical and Dental University, as well as team leader Ichiro Taniuchi of the RIKEN Center for Integrative Medical Sciences, identified a new immunodeficiency (AIOLOS deficiency). They also revealed that this deficiency is caused by a novel pathogenic mechanism involving an abnormal protein that interferes with the function other molecules through forming a complex. In a comment about the research, Professor Morio said, "We believe that there are more diseases caused by heterodimeric interference discovered in this study. If we can develop a drug that specifically suppresses the complex formation of the abnormal protein, it would serve as an effective treatment." Their findings were published in Nature Immunology.
Primary immunodeficiency is a group of genetic diseases in which the sufferers have high incidences of various deadly infections, autoimmune diseases, and cancer development from infancy. It has been reported that 70-80% of the patients worldwide are not accurately diagnosed. There is no cure in many cases, and most radical treatments, even if available, are highly invasive, including hematopoietic cell transplantation.
To date, more than 450 immunodeficiencies have been identified; with the advancement in genetic diagnosis technology, about 20 new disease-causing genes are annually reported. The research group conducted the whole exome analysis of patients with B cell deficiency who did not have any variation in known B cell genes and identified a mutation in the gene encoding AIOLOS.
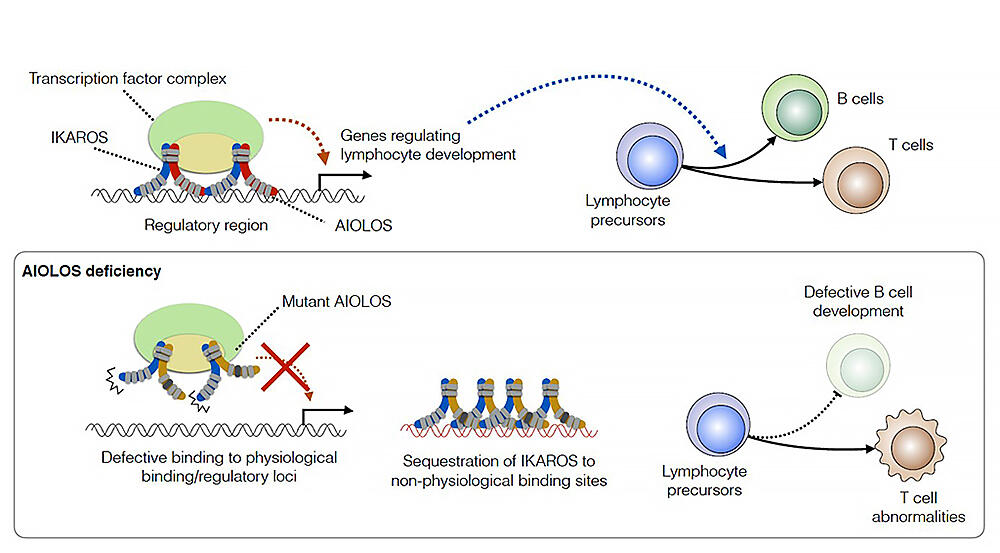
AIOLOS is a transcription factor that regulates lymphocyte development. AIOLOS forms a transcription factor complex in a form of a dimer with IKAROS (heterodimer) to regulate the expression of the genes required for lymphocyte differentiation. Using this knowledge, the research group created a mouse model carrying the AIOLOS mutation corresponding to that in patients (of its 509 amino acids, the one involved in DNA binding was replaced with another amino acid). Analysis of the mouse model showed that mutant AIOLOS interferes with the function of IKAROS by formation of heterodimers, hindering the binding of IKAROS to its target genes, eventually leading to inhibition of B cell differentiation and abnormalities in T cells.
Pathogenic mechanisms of monogenic diseases are classified as loss-of-function, negative dominance, and gain-of-function of the causative genes. In this study, the research group proposed heteromeric interference as a novel pathogenic mechanism of monogenic diseases, where the mutant molecule impairs the function of partner molecules via formation heteromer, rather than the functional defects of its own molecule, leading to disease development. The issue is created by the mutation which sabotages the whole complex. The bad apple spoils the bunch, so to speak.
Therefore, the research group performed gene therapy on the mouse model carrying the mutation corresponding to that reported in patients by additionally deleting the IKAROS-binding domain in AIOLOS. Results showed that this "gene therapy mouse" restored B cell development and T cell abnormalities were also cured. Assistant Professor Yamashita says, "If we develop a molecule that specifically inhibits binding of mutant protein to its partner molecules, it could serve as a therapeutic drug."
Credit: Tokyo Medical and Dental University
There are many diseases likely caused by heteromeric interference. Of the IKAROS mutations known to cause immunodeficiencies and autoimmune diseases, only a handful of mutations can be explained by IKAROS deficiency. On the other hand, more than a dozen mutations are missense variations and may be pathogenic owing to heterodimeric interference. Patients carrying certain IKAROS variants are known to be susceptible to leukemia development. The majority of these variants are also missense variants and thus heteromeric interference may play roles in cancer development. In regard to these finding Professor Morio says, "Heteromeric interference may be responsible for pathogenesis of various diseases, including carcinogenesis and autoimmune diseases, in addition to immunodeficiency."
Therefore, in addition to investigating the function of each molecule, it will be important to pay attention to the functions of protein complexes in future studies. However, performing an integrated analysis of molecules in addition to creating a disease model is challenging for laboratories at ordinary universities in terms of funding and time. Team Leader Taniuchi said, "Researchers and clinicians at Tokyo Medical and Dental University work to identify the cause of the disease in these patients with intractable diseases, and we investigate the mechanism of the disease development using mouse models in RIKEN. Such a network is important." Professor Morio followed up with, "We were able to produce these results because we worked with experts in basic immunology at RIKEN." Going into the future, collaboration is important for both biomolecules and research.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




