The research team led by Hiroshi Kiyono, Professor of The Institute of Medical Science, The University of Tokyo (also a specially appointed professor in Graduate School of Medicine, Chiba University), demonstrated the efficacy and safety of "MucoRice-CTB", a rice-based oral vaccine in which the cholera toxin subunit B (CTB) is expressed as a vaccine antigen within rice seeds.
In the phase I trial, MucoRice administration to healthy adult males markedly increased CTB-specific serum IgG and IgA concentrations in a dose-dependent manner. It was also demonstrated to cross-react with a similar antigen, LTB, indicating that it is useful in preventing not only cholera-related but also travelers' diarrhea. Because this vaccine can be stored at room temperature for a prolonged duration and is effective upon oral administration, it is especially advantageous in developing countries and is expected to be soon approved for practical use. These findings were published in the June 25th, 2021 issue of the international medical journal The Lancet Microbe.
Cholera infection is rare in developed countries such as Japan. However, millions of people are infected with Vibrio cholerae globally every year, with 20,000 to 140,000 deaths reported annually, primarily in developing countries where access to clean water is a challenge. In these areas, mucosal vaccines that do not require refrigerated storage, low-temperature distribution, or generate waste products such as syringes are in demand.
In 2007, Professor Kiyono and his team developed the vaccine-expressing rice "MucoRice", wherein they expressed the vaccine antigen gene (CTB) in rice seeds using genetic recombination technology. The resulting vaccine can be stored at room temperature for a prolonged period and meets necessary criteria such as oral stability and low-cost production. The efficacy of the oral vaccine was demonstrated in experimental animal models such as mice, pigs, and primates. Moreover, the vaccine efficacy was demonstrated in not only diarrhea caused by CT but also travelers' diarrhea caused by heat-labile enterotoxin (LT) from enterotoxigenic Escherichia coli. CT is composed of subunits A and B. CTB is non-toxic.
Credit: Division of Mucosal Immunology, IMSUT Distinguished Professor Unit, The Institute of Medical Science, The University of Tokyo
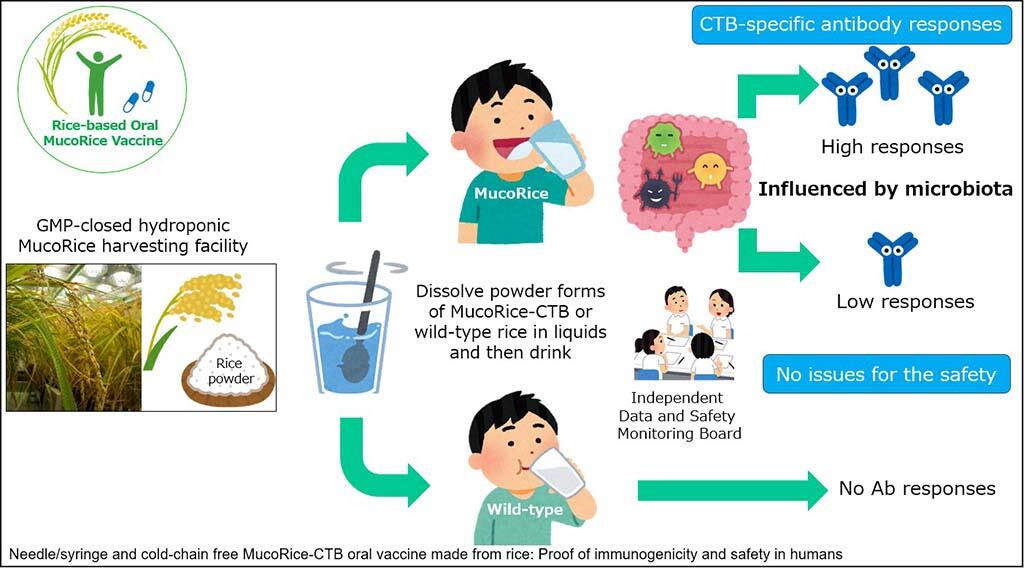
To ensure consistent high quality of MucoRice, the research team established a Good Manufacturing Practice (GMP)-certified closed MucoRice hydroponic cultivation system with Asahi Kogyosha Co., Ltd. and Professor Eiji Goto of the Faculty of Horticulture, Chiba University. Using this system, they conducted a phase I trial for MucoRice at IMSUT Hospital of The Institute of Medical Science, The University of Tokyo from 2015 to 2017. Sixty healthy adult males (aged 20-40 years) without any history of diarrhea were randomly selected for a double-blind, placebo-controlled, randomized controlled trial.
Specifically, they set up a MucoRice-treated and a placebo (wild rice)-treated group. Each of these two groups was further divided into three subgroups receiving different doses (1 g, 3 g, or 6 g) of the respective treatments. Groups receiving the same dose of either MucoRice or placebo were compared. Each subject was orally administered either MucoRice or wild rice four times every 2 weeks. Serum and fecal samples were collected regularly for 2 months after the final oral administration. Serum CTB-specific antibody titer was measured using ELISA. Furthermore, to investigate the effects of MucoRice administration on intestinal flora, metagenomic analysis using next-generation sequencing was performed on bacterial DNA extracted from the fecal samples.
The research team found that CTB-specific IgG and IgA concentrations were markedly increased in the MucoRice-treated group compared with those in the placebo-treated group. Moreover, the vaccine was found to cross-react with LTB, which shows high homology with CTB at the amino acid level. Furthermore, the antibodies induced by oral MucoRice administration had neutralizing effects on CT and LT from enterotoxigenic E. coli in vitro. This means that the vaccine may be useful in preventing diarrhea caused by LT produced by enterotoxigenic E. coli and Shiga toxins.
Metagenomic analysis of bacterial DNA in the fecal samples showed that the intestinal flora is involved in the immune response to MucoRice. Professor Kiyono and his team plan to analyze this involvement in detail in the future.
Professor Kiyono says, "Clinical trials on different races and in developing countries are needed in the future. We must proceed with Phase II and III trials but since public funds alone cannot support these trials, we need the support of pharmaceutical and vaccine companies. As MucoRice is a new vaccine that can be stored at room temperature and administered orally, which makes full use of the rice expression system, we hope to proceed with our research and development, contributing to the SDGs and creating a self-inoculating vaccine that has no harmful effect on the body."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




