A research group including Yuki Matsuki, Nagisa Ohnishi, Yuki Kakeno, Shunsuke Takemoto, Takuya Ishii, Assistant Professor Kazunori Nagao and Professor Hirohisa Ohmiya from the Division of Pharmaceutical Sciences, Graduate School of Medical Sciences of Kanazawa University has developed a revolutionary and eco-friendly chemical process that can generate aromatic radicals using an organic catalyst to cleave bonds between the benzene rings and halogen groups of aromatic halogen compounds.
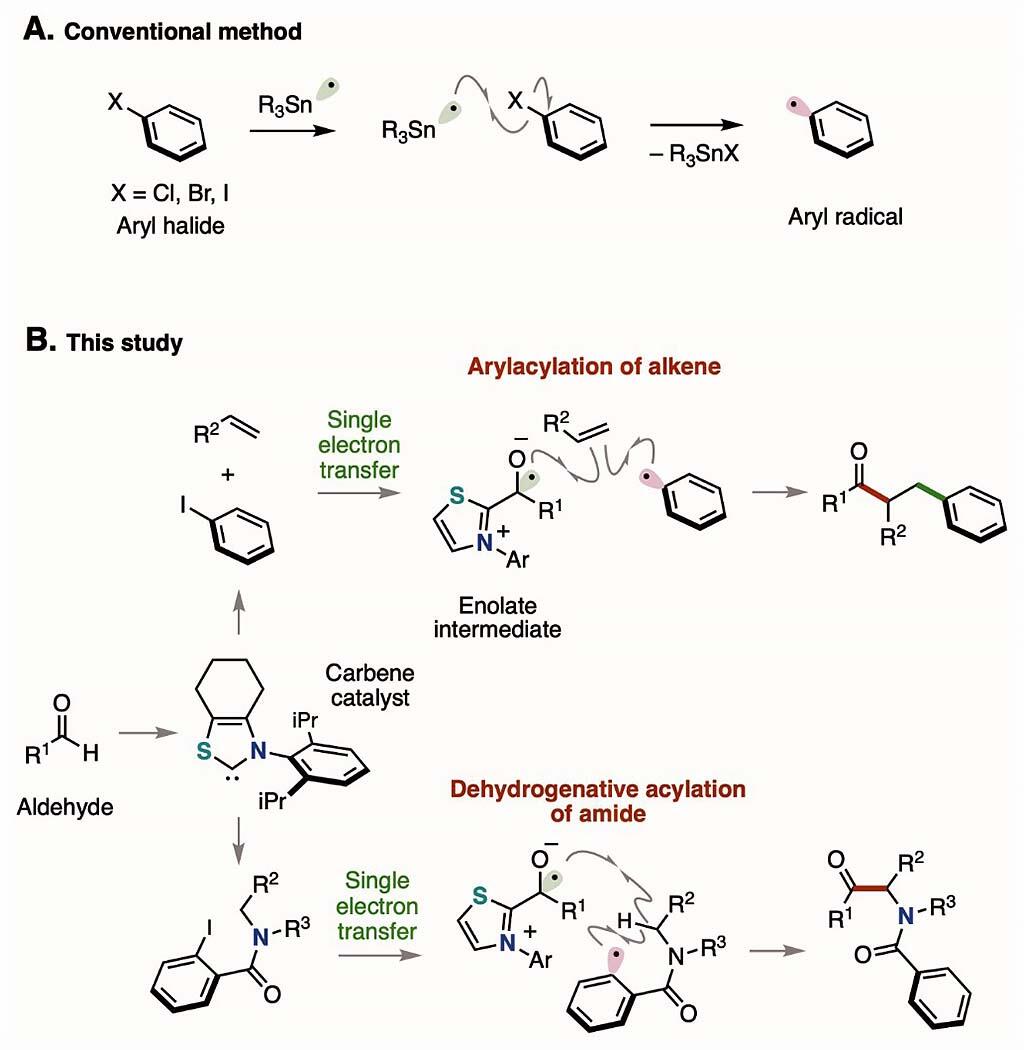
Aromatic halogen compounds are readily available and as a result, metal- and light-catalyzed chemical reactions have been developed to cleave the benzene-halogen bonds in these compounds to generate aromatic radicals. However, because such processes require the use of metal salts and excess oxidizing and reducing agents, chemical processes with lower environmental impact have been needed. Professor Ohmiya said, 'We have developed a chemical reaction that can generate aromatic radicals using carbene (a divalent chemical species with only six electrons ) as an organic (non-metal) catalyst to break the bond between the benzene ring and the halogen group of aromatic halogen compounds.'
The research group used a nitrogen-containing heterocyclic carbene catalyst, which was entirely organic and did not contain metal elements and succeeded both in generating aromatic radicals from aromatic iodine compounds under mild conditions that did not require light or metal salts and in using the resulting radicals for organic synthesis. Specifically, aromatic radicals were produced using two reaction processes, namely the transfer of a single electron from a thiazolium-type nitrogen-containing heterocyclic carbene catalyst to an aromatic iodine compound, with an enolate intermediate consisting of an aldehyde, and the subsequent cleavage of the bond between the benzene ring and iodine group.
Credit: Kanazawa University
The generated aromatic radicals act as a source of the benzene ring, and the bifunctional formation of the alkene, which contains a carbon-carbon double bond, proceeds to yield a ketone in which the benzene ring is substituted. In addition, the dehydrogenation-type acylation reaction of the amide proceeds by using the generated aromatic radicals for the intramolecular hydrogen abstraction reaction, and an α-aminoketone compound is obtained. A substrate with various functional groups can be used in these molecular conversion reactions. The dehydrogenation-type acylation of the amide also facilitated the synthesis of pharmaceutical derivatives. Professor Ohmiya said, 'Aromatic radicals can be easily generated from aromatic halogen compounds that are widely used in organic synthesis using organic catalysts that have a low environmental impact. Therefore, we expect this technological advance to promote the precise assembly of medical pesticides and chemical materials.'
- Organic catalyst: A small-molecule compound that facilitates chemical reactions without being changed itself, does not contain metal elements and contains only organic elements, such as carbon, hydrogen, oxygen, nitrogen, and sulfur.
- Aromatic halogen compound: A compound in which one of the hydrogens of the aromatic (benzene) ring is replaced by a halogen (F, CL, Br, or I) atom.
- Aromatic radicals: Aromatic (benzene ring) compounds with unpaired electrons.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




