Superheavy elements, which have atomic numbers that exceed 103, can be synthesized using an ion beam to induce nuclear fusion. It was thought that the orbits of the outermost electrons would change as the speed of electrons orbiting the nuclei of heavy elements approach the speed of light, thereby causing the chemical properties of heavy elements to deviate from predictions based on the periodic table (a relativistic effect). However, the short lives of such elements have made it difficult to test this hypothesis experimentally.
Post-Doctoral Research Fellow Nadine Chiera (now at the Paul Scherrer Institute), co-principal investigator Tetsuya Sato, and colleagues at the Japan Atomic Energy Agency Advanced Science Research Center succeeded in synthesizing and separating a pure volatile compound of element number 105, dubnium (Db) and found that chemical bonds in the Dubnium-containing compound deviated from expectations based on the periodic table. Regarding this outcome, Sato said, "In this study, we found that Dubnium is less volatile than expected. The relativistic effect manifested by superheavy elements is inherent in all elements. Therefore, we think that this will both improve our understanding of the periodic table and contribute to the sophistication of theoretical chemistry calculations." This work was published in Angewandte Chemie (international version), which is a journal produced by the German Chemical Society and the article was listed on the back cover.
The nuclear synthesis of Dubnium is extremely slow (one atom per 5 minutes), and the resulting atoms have a short lifetime (about 34 seconds). As a result, even 50 years after the element's discovery, the chemical properties of Dubnium remain poorly understood. The research group synthesized Dubnium using the Japan Atomic Energy Agency's Tandem Accelerator and performed chemical synthesis and rapid chemical analysis of the dubnium volatile compounds using an on-line isothermal gas phase chromatography technique that was developed independently. If dubnium possesses the properties indicated by the periodic table, then the volatility of compounds containing Group 5 elements (niobium oxychloride, tantalum oxychloride, and dubnium oxychloride) should be as follows: niobium > tantalum > dubnium. However, this study revealed that the volatility of dubnium was similar to that of tantalum. It was hypothesized that the metallic properties of dubnium (ease of electron emission) are weakened by the influence of a strong relativistic effect and by the compound's non-metallic-like chemical bonds, which share electrons with each other.
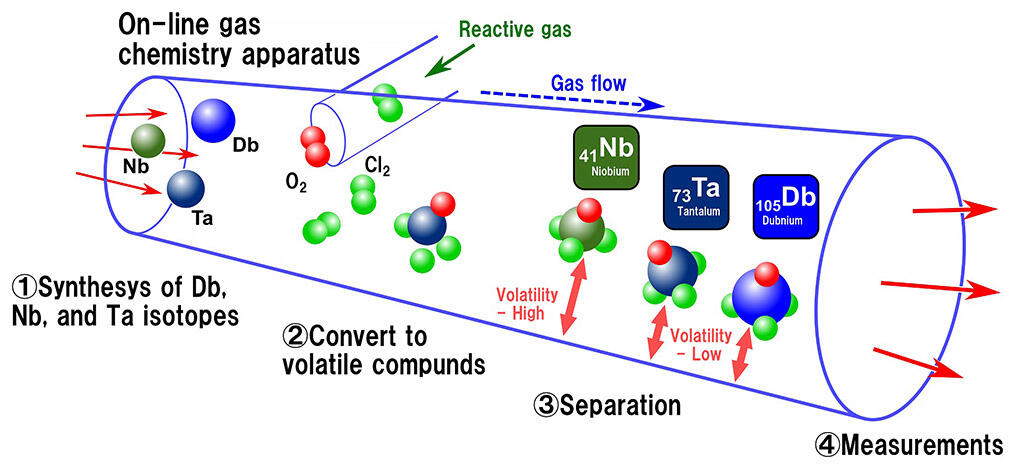
Illustration of the on-line isothermal gas chromatographic experiment for volatile oxychlorides of Db and its homologs, Nb and Ta.
- Nuclear reaction products, synthesized by irradiation of heavy ion beam from the JAEA tandem accelerator, were introduced to the on-line gas chemistry apparatus by a carrier gas flow.
- The nuclear reaction products would react with a reactive gas to form volatile compounds (oxychlorides).
- The volatile compounds would go through an isothermal column made of quartz with many adsorption-desorption with the surface.
- The volatile compounds that reached the end of the column were measured to determine the quantity of the isotopes of interest.
Credit: Japan Atomic Energy Agency
Unlike typical elements, which are naturally occurring, heavy elements are only found under special conditions. However, since the relativistic effect applies to a variety of elements, it is possible to manipulate the electron orbits of elements and compounds and, thereby, to create substances with unprecedented chemical properties. Even though this research is basic, if theoretical chemistry calculations can be advanced through such accumulation, the study's findings are expected to facilitate the design of elements with new functions.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




