Shingo Iwami, Visiting Professor at the Institute of Mathematics for Industry, Kyushu University (currently Professor at the Nagoya University), Shoya Iwanami, Assistant Professor at the Graduate School of Science, Nagoya University, Keisuke Ejima, Assistant Professor at the Graduate School of Public Health, Indiana University, and others have jointly developed a new clinical trial simulator for COVID-19. It is expected to identify the problems pertaining to the clinical trials of antiviral therapy that need to be solved and significantly accelerate the establishment of standard therapies. The findings were published in PLOS Medicine.
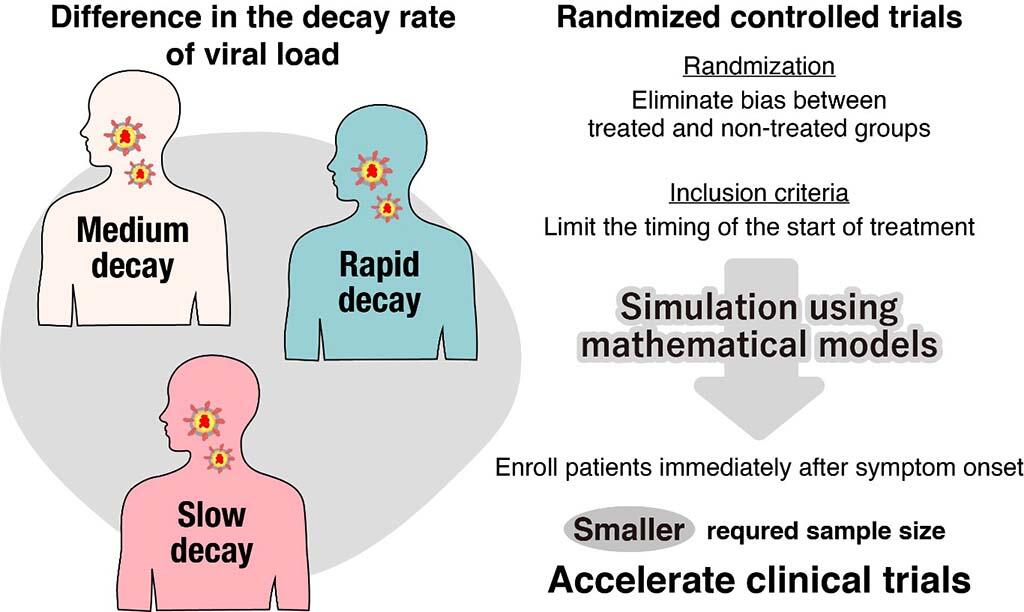
Although several potential antiviral agents against COVID-19 are currently available, few clinical trials have confirmed their therapeutic efficacy. To explore the reasons for this, the research group collected the clinical data of patients and analyzed them using a mathematical model. As a result, they found that there were large differences in the viral dynamics, especially the rate of viral decay, among patients. Differences in the viral dynamics may be the confounding factors that affect the estimation of the treatment efficacy of antiviral agents in observational studies. Conversely, there are randomized controlled trials, which are methods for evaluating the therapeutic effects that are not affected by the confounding factors.
The research group developed a simulator to replicate a randomized controlled trial using a mathematical model. The results revealed that the participation of more than 10000 subjects was required when patients were invited to participate in the study regardless of the time of presentation. This is an unrealistic requirement for sample size, especially in Japan, where the number of infected individuals is limited. Conversely, it was shown for the first time in the world that the required number of subjects can be limited to approximately 500 by allowing only patients who have just developed the disease to participate in the study.
Credit: Shoya Iwanami, Shingo Iwami, interdisciplinary Biology Laboratory (iBLab), Division of Biological Science, Graduate School of Science, Nagoya University
This simulator for clinical trials based on the viral infection dynamics was used in designing doctor-initiated clinical trials and has already been implemented in Japan at an unusually rapid rate. Visiting Professor Iwami said, "In order to quickly establish standard treatment for emerging and re-emerging infectious diseases, it is necessary to design an efficient clinical trial. In this study, a mathematical model using the case of COVID-19 was established. We were able to develop a clinical trial simulator based on this model. Based on this result, we aim to develop a platform for future outbreaks of infectious diseases, including COVID-19."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




