The lungs are made up of the airways and alveoli. The airways have ciliated epithelial cells, which eliminate pathogens and foreign matter from the body using mucus and cilia. To exert this elimination function, ciliated epithelial cells need to undergo coordinated ciliary vibrations. Although airway epithelial cells could be generated in culture using iPS (induced pluripotent stem) cells, it is difficult to reproduce coordinated movement between different cell types in vitro.
Associate Professor Shimpei Gotoh of the Graduate School of Medicine, physician Naoyuki Sone of Kyoto University Hospital, visiting researcher Satoshi Konishi (currently Duke University), Associate Professor Yu-suke Torisawa of the Graduate School of Engineering (currently Healios) at Kyoto University, Professor Sachiko Tsukita of Strategic Innovation and Research Center at Teikyo University and the Graduate School of Frontier Biosciences at Osaka University, and others successfully combined a microfluidic airway-on-a-chip technology with airway epithelial sheets induced to differentiate from human iPS cells. This system reproduced the coordinated ciliary movement between cells in vitro, recapitulating the phenomenon observed in the living body. This research has facilitated the development of a functional evaluation technology. Furthermore, in collaboration with the Departments of Respiratory Medicine and Thoracic Surgery of Kyoto University, the Department of Otorhinolaryngology Head & Neck Surgery of Mie University, and the Department of Pediatrics of the University of Toyama, the research group successfully developed an in vitro model of ciliary dyskinesia, an intractable disease related to ciliary movement caused by gene mutations, recapitulating the condition observed in vivo. Their findings were published in an online issue of Science Translational Medicine.
Associate Professor Gotoh says, "Ciliary dyskinesia is known to be caused by a wide variety of genetic mutations, making its diagnosis difficult. However, the results of this study indicate that establishing iPS cells from the patient's peripheral blood and differentiating them into airway epithelial cells may serve as a useful strategy to perform detailed analyses of this pathological condition. In addition, even novel gene mutations suspected to be the cause of ciliary dyskinesia can be examined for their causal relationship with the disease by comparing with cells that have undergone gene repair. This step may potentially lead to the development of novel treatment regimens."
Ciliary dyskinesia is a hereditary disease caused by the dysfunction of ciliated epithelial cells, but its diagnosis is challenging. Although the disease can be diagnosed through the combination of genetic testing, nasal cavity testing, electron microscopy, and high-speed video microscopy, about 30% of cases yet remain undiagnosed. More than 40 causative genes have been identified to date, and various abnormal patterns have been reported, including attenuation of ciliary movement and loss of coordinated movement, depending on the type of gene mutation.
Accurate diagnosis requires a large number of patient-derived cells, but performing repeated invasive tests is difficult. Thus, the research group established iPS cells from patients' peripheral blood and initiated their study to help ciliary dyskinesia diagnosis and understand the underlying pathophysiology. In 2016, they successfully produced airway epithelial cell sheets from human iPS cells by air-liquid interface culture. However, intercellular ciliary coordination and unidirectional mucus transport could not be reproduced in vitro at that time.
In the present study, the research group cultured airway epithelial stem cells, which had been prepared by a conventional method, in a microfluidic airway chip in the presence of the fluid flow for 14 days to generate airway epithelial cells. The directions of ciliary vibration were aligned, thus forming a unidirectional mucus flow. VANGL1, one of the cell polarity core proteins, was also aligned at the cell boundary opposite to the direction of mucus flow.
When airway epithelial stem cells were immersed in a medium that was not in contact with air during their differentiation, they showed better differentiation into ciliated epithelial cells in the presence of fluid flow than in its absence. Physician Sone says, "Ciliated epithelial cells are present even in the fetus, and the trachea of the fetus, which is filled with the amniotic fluid, has respiratory-like movements. The flow of the fluid in the trachea may also help promote differentiation into ciliated epithelial cells."
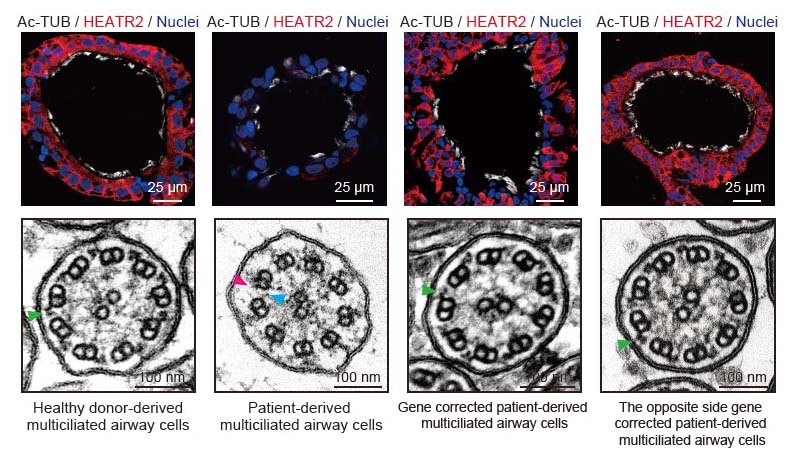
Using iPS cells established from patients with ciliary dyskinesia, the research group generated cells that were repaired for each of two causative gene mutations (HEATR2 and DNAH11) by genome editing, aiming to make a comparison before and after the gene repair. In addition, they generated knockout iPS cells lacking DNAAF2, DNAH11, or RSPH4A, which are the genes known to cause ciliary dyskinesia, to compare with iPS cells derived from healthy individuals.
Credit: Kyoto University
Once established, these iPS cells can be perpetually proliferated and stored. This technique allowed them to use these iPS cells in future studies as needed and repeatedly investigate the cellular pathophysiology in detail by inducing their differentiation into airway epithelial cells. The detailed analysis of their ciliary function indicated that various abnormalities in ciliary movement, which had been sporadically reported with individual causative genes, could be intentionally reproduced even in culture.
It had been previously thought that the vibration of cilia is necessary for coordinated ciliary movement. However, the research group induced differentiation of iPS cells generated from patients with ciliary dyskinesia into airway epithelial cells in the presence of fluid flow, and found that the planar cell polarity protein VANGL1, which indicates the direction of ciliary movement, was oriented in line with the direction of the fluid flow. This means that the cells may be able to cooperate with each other without ciliary movement, which may be useful for clarifying their mechanism of cooperation.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




