Compounds with an endoperoxide structure, such as artemisinin, which is used as an antimalarial drug, are attracting attention as important pharmaceutical resources owing to their potent biological activity. Two types of enzymes are reported to form endoperoxide structures, the first is heme iron-dependent prostaglandin H synthase (cyclooxygenase, COX). The other, fumitremorgin B endoperoxidase (FtmOx1), is a non-heme iron alpha-ketoglutarate (αKG)-dependent dioxygenase derived from Aspergillus fumigatus. Both are oxygenases that introduce two oxygen atoms each into their substrate.
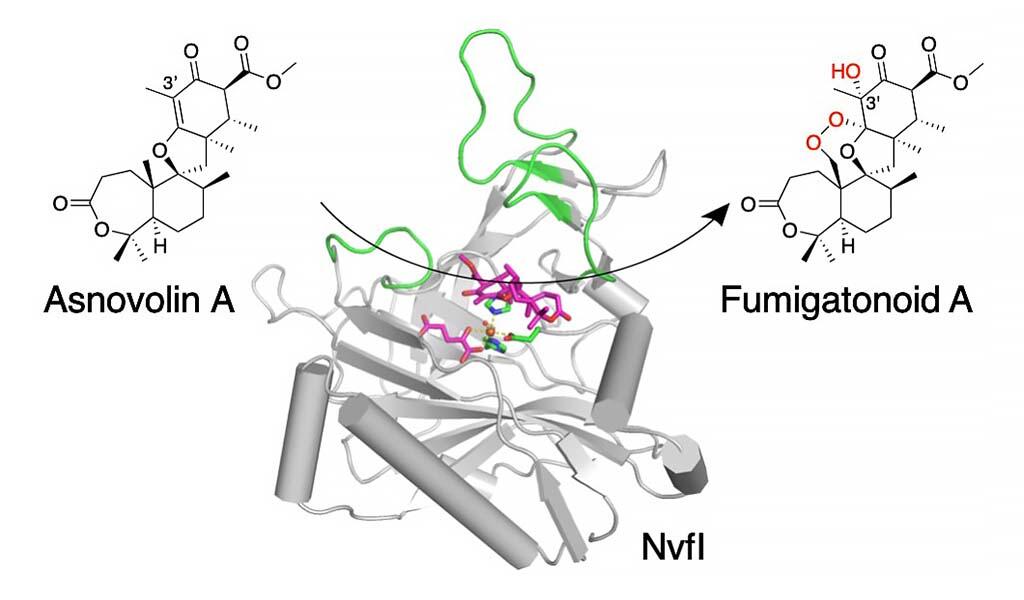
The research group led by Assistant Professor Takahiro Mori, graduate student (at the time of research) Rui Zhai, and Professor Ikuro Abe of the Graduate School of Pharmaceutical Sciences, The University of Tokyo clarified the three-dimensional structural basis of the enzymatic reaction for NvfI, the first reported non-heme iron enzyme, which is involved in the biosynthesis of an endoperoxide-containing natural product derived from filamentous fungi, fumigatonoid A. NvfI simultaneously catalyzes endoperoxide formation and hydroxylation by introducing three oxygen atoms into its substrate in a single reaction.
Precise functional analysis of enzyme reactions using stable isotopes, X-ray crystal structure analysis, and site-specific mutagenesis of the three-dimensional structure revealed that this enzyme simultaneously catalyzes endoperoxide formation and hydroxylation via a reaction mechanism significantly different from those of other endoperoxide-forming enzymes analyzed until date. This work was published online in Nature Communications.
The research group simultaneously introduced three oxygen atoms in the reaction for the biosynthesis of complex skeletal meroterpenoid novofumigatonin derived from the filamentous fungus A. novofumigatus. They analyzed the precise function of the novel non-heme iron enzyme NvfI, which simultaneously catalyzes endoperoxide formation and hydroxylation, and clarified the reaction mechanism. The non-heme iron enzyme NvfI uses asnovolin A as a substrate and introduces three oxygen atoms, including an endoperoxide structure and a hydroxyl group at the C3' position, to catalyze the formation of fumigatonoid A.
First, the researchers performed an enzymatic reaction using water or oxygen containing stable isotopes and showed that NvfI introduces three oxygen atoms into the substrate simultaneously in one round of the enzymatic reaction. Furthermore, to clarify the detailed catalytic mechanism of NvfI, the X-ray crystal structure of the enzyme was analyzed. The complex apo structures of NvfI and the substrate were successfully obtained. Comparison of the active site structure of the apo form of the complex revealed significant conformational changes involving multiple amino acid residues. The findings indicated that substrate binding causes large dynamic changes in the active site structure.
Credit: The University of Tokyo
In the enzymatic reaction mechanism of COX and FtmOx1, the tyrosine residue in the active site plays an important role in endoperoxide formation by yielding tyrosine radicals. By introducing site-specific mutations into the amino acid residues of the active site based on the obtained complex structure, the role and importance of each amino acid residue constituting the active site were investigated. The results suggest that NvfI does not utilize amino acid residues, such as tyrosine residues, in the active site to mediate elimination and donation of hydrogen atoms in the catalytic mechanism. The findings clarified that the catalytic mechanism of NvfI underlying endoperoxide formation is significantly different from that for the reaction of COX and FtmOx1.
In this study, the researchers reported the first oxygenase that simultaneously introduces three oxygen atoms into its substrate in a one-step enzyme reaction--NvfI. The structural basis of the enzyme reaction was clarified by precise functional and three-dimensional structural analyses of the enzyme. Many untapped enzymes exist in nature whose functions have not yet been clarified. It is expected that they include enzymes that catalyze reactions that go beyond our conventional understanding of organic chemistry. Advances in pharmaceutical science are expected through discovery of such enzymes, clarification of their functions, and their development into biocatalysts and for application in synthetic biological methods.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




