Associate Professor Masazumi Tamura of the Research Center for Artificial Photosynthesis, Osaka City University, Professor Keiichi Tomishige of the Graduate School of Engineering, Tohoku University, Chief Kenji Nakao of Advanced Technology Research Laboratories, Nippon Steel Corporation, and others have succeeded in the development of world's first catalytic process that directly synthesizes polycarbonate diols from atmospheric carbon dioxide and diols without using a dehydrating agent. They also showed that a polycarbonate diol could be synthesized with high yield and high selectivity by combining a cerium oxide catalyst with the process. This work was published in the journal "Green Chemistry".
Climate change and natural disasters associated with global warming are becoming increasingly prevalent, and there is a global need to reduce carbon dioxide. To combat this issue, research efforts have been devoted to the conversion of CO2 into useful chemical products, considering carbon dioxide as a C1 chemical raw material. However, carbon dioxide is a very stable molecule with low reactivity, and its activation depends on the use of a highly functional catalyst as well as good process design.
There are non-reductive methods that convert carbon dioxide without changing the oxidation state of carbon in carbon dioxide conversion technologies. If alcohols and diols can be successfully reacted with carbon dioxide, useful chemicals such as organic carbonates and polycarbonates can be produced. These carbonate compounds are mainly synthesized using toxic chemical raw materials such as phosgene and carbon monoxide, making it imperative to develop technologies to replace these processes.
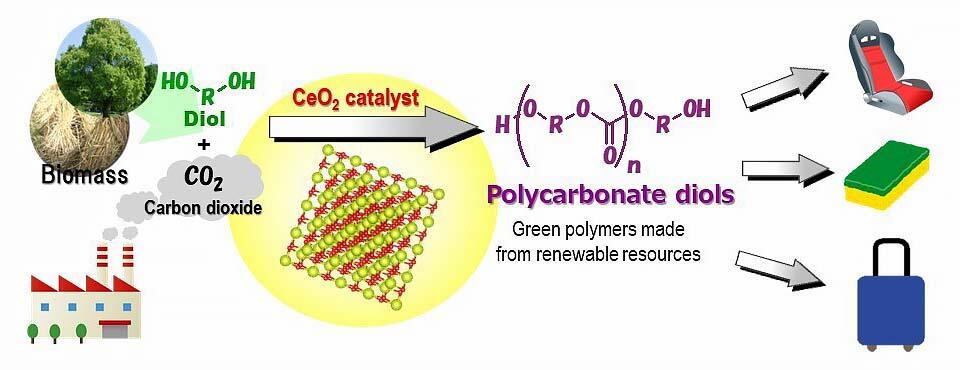
For the long-term immobilization of carbon dioxide, it may be advantageous to explore the conversion of CO2 to a polymer. However, in the synthesis of polycarbonate from carbon dioxide and diols, the reaction hardly proceeds by mixing the reactants, and the amount of the desired polycarbonate is negligible unless the water by-product is removed (approximately 1%). As an example of direct polymerization from carbon dioxide and diol, a catalytic reaction system using nitrile as an organic dehydrating agent and cerium oxide as a catalyst has been reported. However, high-pressure carbon dioxide is required, and the recovery and regeneration of the dehydrating agent are challenging. Another drawback is that nitrile, which is a dehydrating agent, reacts with the raw material diols and the products, and that the by-products are entrapped in the target product. This has necessitated the development of a catalytic process that does not use a dehydrating agent. The research group focused on the difference in boiling point between the products, diols, and water, to develop a water removal method that does not use a dehydrating agent. They predicted that the desired carbonate synthesis would proceed by via gas stripping of carbon dioxide at normal pressure, followed by evaporating and removing water.
In the experiment, carbon dioxide at normal pressure was blown into 1,6-hexanediol and triethylene glycol dimethyl ether using cerium oxide as a catalyst, and the reaction was carried out at 210 °C. As a result, polycarbonate diol was formed with a yield of 92%, and water, the by-product, was evaporated and removed. It is a very simple catalytic reaction system. This system is considered to be applicable to substrates whose boiling point is sufficiently higher than that of water. It can also be used to synthesize organic carbonates, carbamates, and ureas, which are useful as additives for lithium-ion batteries and/or raw materials for polymer synthesis.
Through the establishment of various synthesis routes, it is expected that a catalytic process will be developed that contributes to the chemical immobilization of carbon dioxide. According to Associate Professor Tamura, "Reducing carbon dioxide in the atmosphere is a global priority, and the chemical immobilization of carbon dioxide is a potentially effective method for aiding in this goal In this study, we succeeded in developing a new solid-catalyzed process that can directly convert atmospheric carbon dioxide into a polymer. By reacting carbon dioxide with a diol in the presence of a cerium oxide catalyst, polycarbonate diol, which is useful as a chemical product or raw material for chemical products, can be obtained without using a dehydrating agent. In the future, we will explore further improvements of the catalyst and reaction process for practical use."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




