A research group comprising Kota Nomura, doctoral student at the Graduate School of Science, Osaka University, Assistant Professor Yuta Maki, Lecturer Ryo Okamoto, Professor Yasuhiro Kajihara, and Associate Professor Ayano Satoh of the Graduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, has developed a glycoprotein synthesis method through a completely unprecedented approach that employs thioacid as a key substance. They achieved chemical synthesis of sugar chain-bound C-C motif Chemokine Ligand 1 (CCL1) and interleukin 3 (IL3), which are important cytokines in the immune response. In addition to this, in cell experiments, they were able to analyze the function of sugar chains during the binding of IL3 synthesized by this method to IL3 receptors on hematopoietic cells.
This ability to incorporate sugar chains into proteins at will is expected to clarify the function of sugar chains in glycoproteins. Through this it will also become possible to improve the efficiency of synthesis of glycoproteins and glycopeptide, which are medium-high molecular weight drugs. These findings were published in the Journal of the American Chemical Society.
Glycoproteins have a variety of functions, including immune responses to viruses, inflammatory responses, and signal transduction to cells. However, the sugar chains that bind to proteins in the body are structured in a variety of ways, making it difficult to identify the important structures of the chain. It is also extremely difficult to obtain high-purity glycoproteins using current biotechnological methods. One of the solutions to this problem is chemical synthesis, wherein a sugar chain structure is freely altered, and a high-purity glycoprotein is obtained. However, conventional glycoprotein synthesis methods require as many as 100 conversion steps. This means that in general, it is difficult to synthesize glycoproteins.
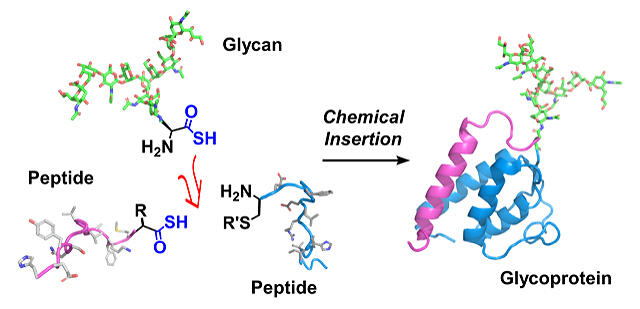
As a solution to this, and in order to establish an efficient synthesis method for glycoproteins with uniform structure, the research group have developed chemical syntheses of glycoproteins with various sugar chain structures using sugar chains isolated from chicken eggs. As the most difficult and efficient molecular construction method, a synthetic method involving insertion of a sugar chain into a protein has been considered for a long time. However, as proteins and sugar chains have various functional groups, it has been challenging to precisely control and link their molecular structures in small molecule drugs. A highly controlled, chemically selective reaction was required. By using thioacid as a key substance, the research group has developed a novel amide-binding reaction "diacyl disulfide coupling (DDC)" that chemically and selectively connects the sugar chain asparagine thioacid to the protein (peptide) chain. By employing the DCC synthesis strategy, a glycoprotein synthesis method was developed where the carbohydrate chain can be freely inserted in any position of the protein.
Through this method the synthesized sugar chains can be rapidly incorporated into glycoproteins, and high-purity glycoproteins could be successfully synthesized from raw materials in only five or so steps. By measuring the effect of IL3 on the growth of cells with synthesized sugar chains using these methods and evaluating the function of the sugar chains, the researchers were able to develop a new mechanism of action that facilitates the binding between IL3 and its receptor proteins. Rapid procurement of high-purity glycoproteins with uniform sugar chains is required for investigating the effects of such sugar chains. This synthesis method will facilitate further research on the functions of sugar chains on glycoproteins in the future. Furthermore, using this synthetic method, glycoproteins can be quickly prepared and used in pharmaceutical glycoprotein preparations.
"Chemical synthesis of glycoproteins started about 20 years ago. At that time, it was not very practical and we were hardly able to synthesize glycoproteins. This current synthetic research began being conducted in 2015 by Mr. Nomura, Mr. Haraguchi, a graduate student and Dr. Okamoto and Dr. Maki, who are university staff, and developed from the discovery of an unexpected peptide synthesis method. Although improvements are still needed, glycoproteins can be synthesized in just a few steps. This is a good result and I think it will be useful in the field of drug discovery research in the future," said Professor Kajihara.
Credit: Osaka University
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




