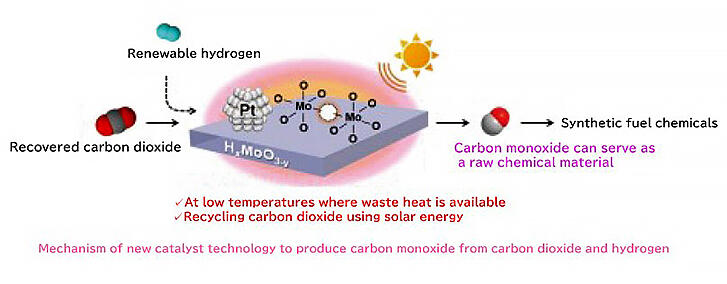
Carbon monoxide (CO) is hazardous to humans and other living organisms. However, it is extensively used as a raw material for manufacturing liquid hydrocarbons such as alcohol, gasoline, and jet fuel in the chemical industry. If CO2, one of the primary contributors to global warming, can be reduced to CO, it will be possible to produce useful substances while reducing emissions, achieving two objectives simultaneously. However, in conventional technology, inefficiency is a primary concern, as a high temperature of 500 °C or more is required to react CO2 with hydrogen (H2) and obtain CO.
A research group led by Associate Professor Yasutaka Kuwahara of the Graduate School of Engineering, Osaka University, has succeeded in selectively generating CO from CO2 using a catalyst wherein fine platinum particles are supported on molybdenum oxide, allowing the reaction to proceed efficiently at approximately 140 °C, a much lower temperature than that reported earlier.
The research group also found that this catalyst is effective for deoxygenation reactions, whereby oxygen atoms are removed from compounds containing oxygen, and that the reaction rate is significantly improved by approximately four times when the catalyst is irradiated with light. This is due to surface plasmon resonance, which causes the free electrons in molybdenum oxide to resonate with light of a specific wavelength, promoting the reduction reaction. If this catalyst technology is applied, it will be possible to use waste heat from factories and light energy (e.g., sunlight) to recycle CO2 with low power consumption. The researchers hope that this discovery will contribute to addressing concerns that affect the society.