Adenosine triphosphate (ATP) is a metabolite involved in the storage and utilization of energy to support living systems. Recent studies have shown that ATP suppresses amyloid fibril formation in proteins, but it was unclear how ATP stabilizes proteins. A research group, consisting of Mayu Nishizawa, a doctoral student at the Graduate School of Engineering, Kyoto University (at the time of research); Assistant Professor Daichi Morimoto, Professor Masahiro Shirakawa, Associate Professor Kenji Sugase, and Assistant Professor Erik Walinda of the Graduate School of Medicine, Kyoto University; and Dr. Benjamin Kohn, Researcher, and Director Ulrich Scheler, both of the Leibniz Institute of Polymer Research, has successfully used nuclear magnetic resonance (NMR) to show that ATP at the same concentration as found in the cell interacts in a weak and non-specific way with proteins, and that ATP self-associated. Associate Professor Sugase said, "It is thought that by self-associating, ATP can bind to the hydrophobic parts of proteins, causing them to stabilize. The amount of ATP in the body decreases with age. I think that drugs and supplements that suppress this decrease in ATP may suppress amyloid fibrosis and prevent the associated neurodegenerative diseases." The findings of this study were published in the Journal of the American Chemical Society.
Individual intracellular protein concentrations are around 0.01 mM, whereas ATP is present at high intracellular concentrations ranging from 5 to 10 mM. ATP is referred to as the energy currency of life because it releases energy when one of its phosphate groups is released. ATP has also been shown to have a function in stabilizing proteins and suppressing amyloid fibrillation, but it was unclear how ATP interacts with and stabilizes proteins.
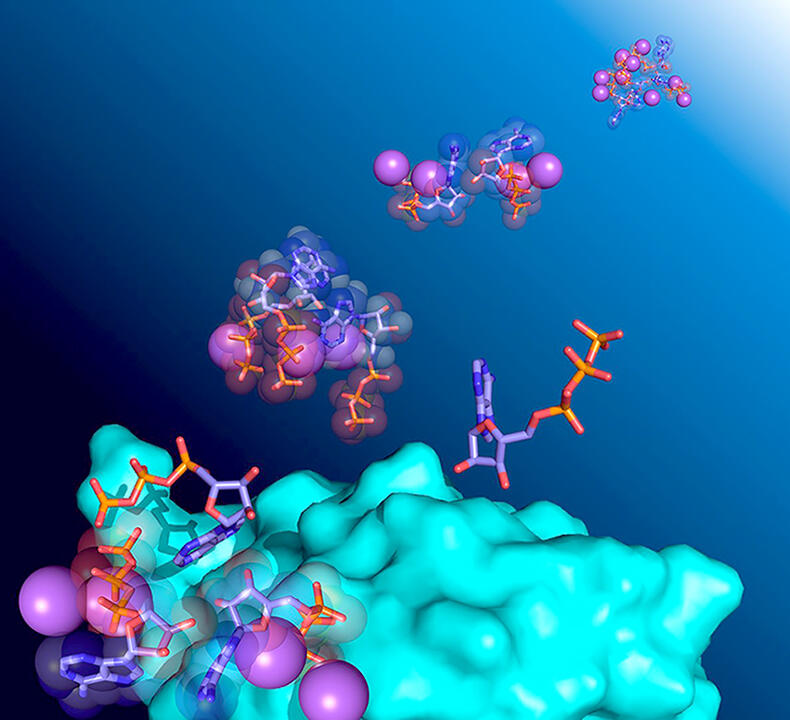
The research group believed that ATP had been overlooked in the past because the stabilizing interactions that it has with proteins was considered too weak. This interaction between ATP and proteins was analyzed using NMR, which can detect even very weak interactions at the atomic level. The researchers analyzed several targets, including α-synuclein, which forms amyloid fibrils; the very stable protein, ubiquitin; and p62, which undergoes dimerization. First, ATP was gradually added to a protein solution that was prepared at a concentration similar to that in the cell (50 µM), and the NMR data was measured over time. The results showed a slight shift in the NMR signal of the protein. This means that ATP interacts very weakly with the protein.
The way in which this particular chemical shift occurred was different from conventional understanding. The shift was hardly noticeable at low concentrations, but when the concentration was high, a large shift was observed. It is known that the effect of ATP on stabilizing proteins appears from a concentration of approximately 5 mM, and a large shift was observed at approximately 5 mM.
Next, dynamic light scattering measurement, a diffusion coefficient measurement, and calorimetry were all performed. Dynamic light scattering showed that the apparent molecular size increased when the concentration of ATP increased. Diffusion coefficient measurements showed that there were only few components in the ATP solution that self-assembled and slowed diffusion. Calorimetry detected heat absorbed when self-association broke down.
The researchers also investigated how ATP self-associates using molecular dynamics calculations. Through this it was proven that various associations existed, and it was clarified that ATP self-associates more frequently as its concentration increases. The researchers also noted that the presence of ATP resulted in a higher NMR signal for some of the proteins. The NMR signal they observed was that of the amide group of the protein, but the hydrogen atom of the amide group easily exchanges with water molecules. In general, this hydrogen exchange reduces the NMR signal.
Credit: Kyoto University
From this outcome the researchers considered the idea that ATP might be slowing the hydrogen exchange rate between the amide group and water. They then performed analyses to investigate this phenomenon. For example, in the presence of ATP, the rate of hydrogen-exchange decreased at the Gly75 residue of ubiquitin. In other words, it can be interpreted that ATP surrounds the protein, preventing water molecules from accessing the protein. Proteins have regions that are easily adsorbed to other molecules. Because ATP surrounds the entire protein, including these regions, it can stabilize the protein. As these results were common to the three proteins analyzed, they can be assumed to be general properties of ATP.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




