A research group including Professor Etsuko Miyagi and Professor Hisashi Hirano of Yokohama City University Association of Medical Science and Visiting Associate Professor Noriaki Arakawa of the Advanced Medical Research Center (also serving as Senior Researcher at the National Institute of Health Sciences) announced on July 28th that, In collaboration with Tosoh (President and CEO Toshinori Yamamoto), they have developed a new diagnostic marker for ovarian clear cell carcinoma. Proteomic analysis revealed that ovarian clear cell carcinoma produces the protein tissue factor pathway inhibitor 2 (TFPI2). Using this knowledge, a new diagnostic marker was developed through collaboration with Tosoh. This new development is expected to lead to higher diagnostic performance and more effective treatment for the disease through combined use with existing biomarkers. The group's results were published in the International Journal of Clinical Oncology.
Epithelial ovarian cancer affects approximately 10,000 people a year in Japan and 4,500 of those affected succumb to the disease. It is classified into four major histological subtypes, including serous, clear cell, endometrioid, and mucinous epithelial ovarian cancers and clear cell carcinoma makes up 25% of cases. Clear cell carcinoma develops from endometriosis, a disease involving benign growths of endometrial cells outside of the uterus. The incidence of the disease in younger age groups has been trending upwards, and there are many cases of chemoresistance resulting in poor prognosis. There are also many cases with negative or low levels of existing ovarian cancer biomarkers. The existing biomarker CA125 is able to detect ovarian cancer with high sensitivity but struggles to selectively diagnose clear cell cancer even when combined with other diagnostic markers. This meant that there was a need to identify a new, highly specific biomarker for detecting clear cell carcinoma.
A research center was established at Yokohama City University with more than a dozen mass spectrometers with the support of the "Project for Developing Innovation Systems" run by the Japanese Ministry of Education, Culture, Sports, Science and Technology. In the early phase of the project, proteins derived from clear cell cancer were investigated by comprehensively analyzing the blood samples of patients using these mass spectrometers. However, blood contains albumin and globulin (derived mainly from the liver) at concentrations approximately 1 billion times higher than those of cancer-derived proteins, making detection difficult. To investigate further, the researchers focused on several ovarian cancer-derived cell lines established by the Department of Obstetrics and Gynecology.
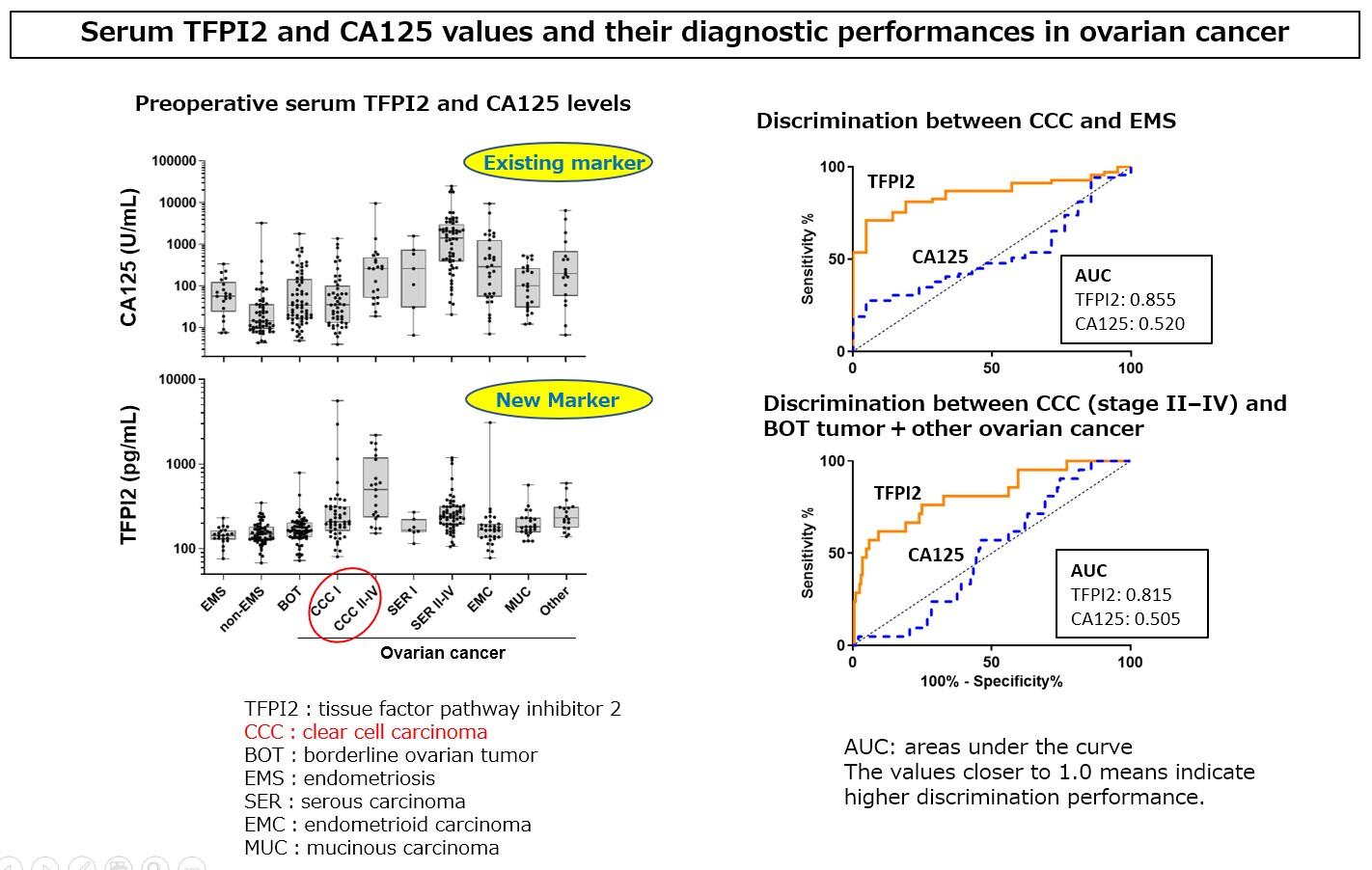
Fig. 2. (right) Receiver operating characteristic curves and areas under the curve (AUC) values of tissue factor pathway inhibitor 2 (TFPI2) and serum CA125 in discriminating clear cell carcinoma from other ovarian lesions. A; in patients with Stage II¬-IV clear cell carcinoma vs. borderline ovarian tumor+ epithelial ovarian carcinoma, B; clear cell carcinoma vs. ovarian endometriosis, C; clear cell carcinoma Stage I vs endometriosis, D; clear cell carcinoma Stage II-IV vs. endometriosis. TFPI2, tissue factor pathway inhibitor 2, CCC, clear cell carcinoma; BOT, borderline ovarian tumor; EOC, epithelial ovarian carcinoma; EMS, endometriosis.
These culture media were subjected to proteomic analysis using a mass spectrometer, and 891 proteins from clear cell carcinoma, 1085 from mucinous carcinoma, and 948 from serous carcinoma were identified. Among them, 148 proteins were found to be expressed only in clear cell cancer, and one of them, TFPI2, was identified as a novel ovarian cancer biomarker. TFPI2 is a protease inhibitor that is expressed in the placenta and was first discovered by Professor Miyagi's research group in 1993 during their search for bioactive substances secreted by cancer cells.
The next step the group took was the development of a measurement reagent that detects TFPI2 levels in the serum through the use of two monoclonal antibodies. This was done in collaboration with Tosoh. Detection of TFPI2 can be achieved in 20 minutes using this reagent and a fully automated enzyme immunoassay (AIA analyzers). To verify its performance, the concentration of TFPI2 in the serum was measured and diagnostic performance was compared against the existing marker, CA125, in 425 gynecological patients, including clear cell cancer patients, at Yokohama City University and Nara Medical University. As a result, it was confirmed that high concentrations of TFPI2 were detected in the serum of clear cell carcinoma patients. Due to its differences when compared to existing markers, TFPI2's feasibility as a new diagnostic marker was confirmed.
Clinical trials aimed at practical application of the marker were held at 5 medical institutions in Japan (Yokohama City University, Nara Medical University, Kanagawa Prefectural Cancer Center, Hyogo Prefectural Cancer Center, and Shizuoka Prefectural Cancer Center). Measurement of preoperative levels of TFPI2 in the serum of 351 patients with ovarian tumors confirmed that clear cell cancers specifically exhibited high levels of the protein. The high specificity of TFPI2 in determining clear cell carcinoma in these 274 ovarian cancer patients demonstrated its usefulness in a clinical setting. It was also confirmed that testing for the combination of TFPI2 and CA125 substantially improves the sensitivity of detection of stage I (early) clear cell carcinoma.
As a result of these findings, TFPI2 was approved for manufacturing and sales as an in vitro diagnostic test for the aid of ovarian cancer diagnosis, and this kit received insurance coverage in April 2021. It also became available in a clinical setting from the end of July. Ovarian clear cell carcinoma is relatively common among Asians, and unlike other gynecologic disorders, has chemoresistance to many of the current anticancer drugs. As a result, these findings are expected to assist clinicians in decision-making for interventions, such as surgery, by using it to discriminate between cases with negative or low CA125 levels or in benign endometriosis. In the future, the research group plans to continue with the aim of clarifying the production mechanism, focusing on changes in the levels of TFPI2 after surgery, as well as before and after anti-cancer treatment. They also plan to look at the process that leads to malignant tumor formation from benign endometrial cysts.
Regarding this outcome, Professor Miyagi commented, "Using TFPI2 allows us to prepare in advance for extended surgery. This is an advantage because, as doctors, we aim to treat clear cell carcinoma effectively through removing the cancer during the initial surgery. Of course, we believe that this finding will also help our patients receive better treatment, improving their quality of life, and will also lead to personalized medicine in the future."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




