As the number of patients with Alzheimer's disease (AD) continues to grow in Japanese society, which faces an aging population, identification of the mechanisms underlying the onset of the disease is an important agenda. Two types of amyloid-β proteins (Aβ) are found in the AD brain. Of these, Aβ42, which is a protein made of 42 amino acids linked together, easily forms fibrillar aggregates and is believed to be more closely associated with the onset of AD. Previous studies have revealed the structure of fibrillar Aβ42 aggregates synthesized in vitro. However, because the amount of fibrillar Aβ42 aggregates that accumulate in the brain of AD patients is very limited, the actual structure of the Aβ42 aggregate is not well understood.
In this study, an international research group led by Professor Yoshitaka Ishii of the School of Life Science and Technology, Tokyo Institute of Technology, endeavored to detect the difference in molecular structures of fibrillar Aβ42 aggregates between synthesized aggregates and patient aggregates using a measurement method called "high-field solid-state nuclear magnetic resonance (NMR)". They succeeded in performing NMR spectrum analysis of the sample within only 9 minutes. This high-field solid-state NMR method is a new measurement method developed as an advanced application technology for 1.3 gigahertz ultra-high magnetic field NMR equipment using the high-temperature superconducting wire joint technology currently under development through the JST-Mirai Project. Significant improvements in NMR-based analytical sensitivity enabled this successful structural analysis.
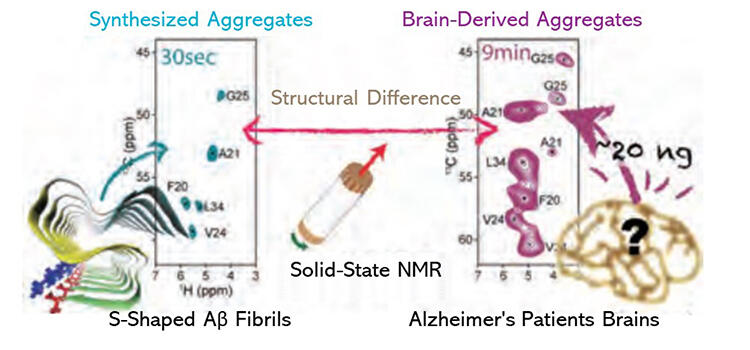
This result promotes further understanding of the molecular structure and function of fibrillar Aβ42 aggregates derived from the brains of AD patients, and the analysis method may be applied to development of therapies such as antibody medicine.




