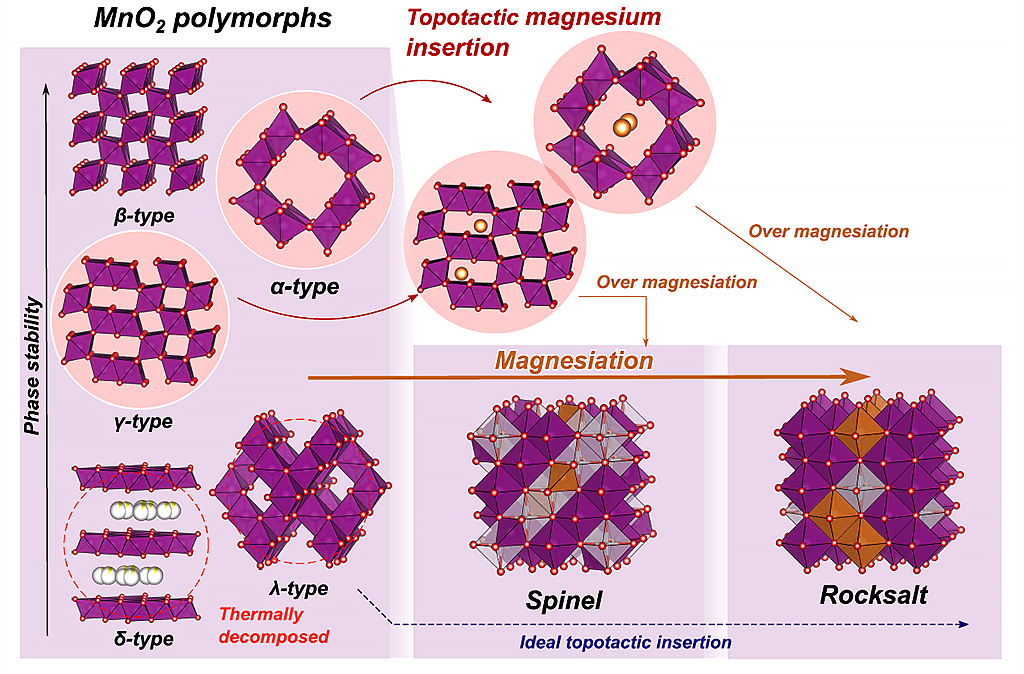
Manganese dioxide (MnO2) is a common material employed as the cathode in alkaline and lithium batteries. In addition to the γ-type crystal structure, which has been widely used as a battery material, there are different crystal polymorphs with the same composition, such as α, β, δ, and λ types. The research group led by Takuya Hatakeyama (a graduate student), Professor Tetsu Ichitsubo, and other members from the Tohoku University Institute for Materials Research, in collaboration with Maximilian Fichtner, a professor at the Helmholtz-Institute Ulm, Karlsruhe Institute of Technology, Germany, highlighted that electrochemical reactions at moderate temperatures can be employed to understand reactions that cannot be sufficiently observed at room temperature due to the diffusion ratio. The research group found that Mg ions can be inserted into the MnO2 polymorphic structure; furthermore, this process is reversible and does not destroy the matrix structure (it is a topotactic reaction). This discovery is expected to accelerate the development of magnesium storage batteries with an MnO2-based material as the cathode.
Multivalent ions, such as Mg ions, move much slower than monovalent ions inside the solid cathode. In Mg insertion experiments at room temperature, Mg ions tend to concentrate locally on the surface of the cathode MnO2 particles. At high Mg concentrations, the MgO rock salt phase, which is the most thermodynamically stable phase, is formed non-uniformly on the particle surface. The intrinsic Mg ion insertion/removal characteristics of MnO2, which determine if Mg ions can be inserted, have not been clarified. Moreover, considering that the Mg ion insertion may induce structural changes, another consideration was whether the matrix structure could be maintained when Mg ions were inserted.
In the study, five crystal polymorphs of MnO2 with α, β, γ, δ, and λ-type structures were used. The insertion of electrochemical Mg ions at a high temperature (150 °C) promotes diffusion in the solid, which enables uniform distribution of Mg ions throughout the manganese dioxide particles. Consequently, the phase transformation behavior of the MgO polymorphs, which could not be observed at a controlled diffusion rate and room temperature, was observed. Among the polymorphs, the λ-type MnO2 has a structure in which all Mg atoms have been removed from the extremely stable MgMn2O4 spinel compound; however, it is extremely unstable from an energy standpoint. However, at 150 °C, the λ-type structure was the only structure capable of accommodating Mg in high concentrations under ideal topotactic insertion.
Essentially, the findings of this experiment reveal that there are two types of structure (α-type and λ-type) that maintain robustness while allowing the insertion and desorption of Mg ions. It was found that the initial structure was maintained even when the Mg ions were inserted at ~200 mAh/g for the α-type MnO2 and ~100 mAh/g for the γ-type MnO2. It was shown that the α-type MnO2, which is metastable and structurally resistant to the chemical forces that promote structural transformation to the stable phase (driving force of transformation), can periodically remove Mg ions at ~100 mAh/g.
(provided by Tohoku University)
According to Professor Ichitsubo, "The metastable structure tends to irreversibly transform into a rock salt structure when Mg is inserted. Conversely, without Mg, problems that hinder practical application of the material, such as the high instability of the matrix structure, were discovered. In the future, by promoting the development of materials with manganese dioxide as the starting material, the irreversible structural changes at the poles can be suppressed. Furthermore, the research group will continue to search for cathode materials that are highly compatible with high-performance electrolytes at room temperature and are not limited to functioning at moderate temperatures."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




