Professor Isao Azumaya, Research Associate Shoko Kikkawa, and their research group at the Toho University, Faculty of Pharmaceutical Sciences, demonstrated that optically inactive indolyl sulfonamides exhibit chiral crystallization to afford high proportions of optically active crystals, depending on the type and combination of specific intermolecular interactions.
The formation of an optically active crystal from an optically inactive solution or a molten state is called chiral crystallization. An interesting aspect of the phenomenon is that this optically active state can be obtained only via crystallization. This phenomenon is observed in 5% to 10% of all compounds whose solutions are optically inactive. However, the mechanisms behind chiral crystallization are still elusive.
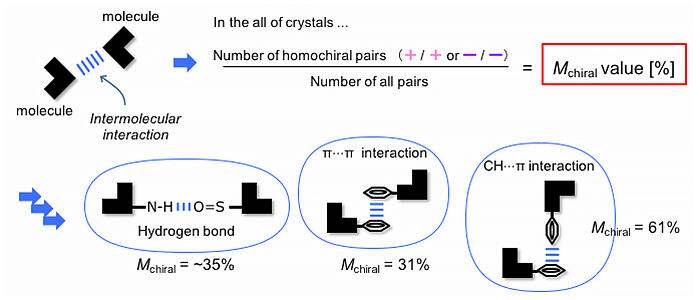
A comprehensive investigation of the relationship between the type of substituents and the optical activity of the aromatic sulfonamide derivative crystals revealed that derivatives with an indolyl skeleton on the sulfonamide nitrogen side underwent chiral crystallization at unusually high rates. In the process, they focused on the mutual chirality of molecules associated by intramolecular interactions. The proportion of molecules with the same chirality and linked by interactions was denoted as Mchiral. The Mchiral values of the various intramolecular interactions were determined.
It was found that a combination of specific intermolecular interactions resulted in an abnormally high rate of chiral crystallization, and this was not observed when the interactions were present alone. In addition, by collecting several data points (X-ray structure analysis and optical activity data) on infrequent events, which cannot be conclusively established from a small number of data points, it is possible to express the phenomenon in terms of comparable numerical values (i.e., a ratio).
Credit: Toho University
There are no methods that comprehensively investigate the optical activity of crystals using Mchiral as an index and that associate the proportion of compounds affording optically active crystals with the structure of the molecule. It was suggested that the application of this method would enable the (intentional) design of compounds undergoing chiral crystallization, a phenomenon that was discovered by chance. Professor Azumaya stated, "It is expected that this method will be developed from various perspectives, such as the fabrication of optically active crystalline materials that can be obtained only by crystallization, development of asymmetric synthesis methods by fixing the chirality in the obtained crystal, and understanding the origin of chirality seen in life."
■ Optical activity: The ability to rotate a vibrating surface when linear (planar) polarized light passes through molecules in an aggregated state, such as a solution, molten state, or crystal. Optical inactivity is the case where light passes through a vibrating surface without rotating it.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




