Hiroshi Sato, the leader of the Emergent Molecular Assembly Research Unit of the Center for Emergent Matter Science at RIKEN, and others, in collaboration with Post-Doctoral Research Fellow Wenjing Meng and others of the School of Engineering, the University of Tokyo, announced on October 14th that they have succeeded in developing elastic crystals that respond to external forces by precisely arranging catenanes which are molecules in which the ring structure is mechanically interlocked in a three dimensional fashion. Upon investigating the crystal structure through single-crystal X-ray diffraction analysis, it was found that, although the structure is crystalline, it changes its shape when an external force is applied and returns to its original shape when the force is removed similar to rubber. The crystal is expected to be applied as a porous material that can absorb and desorb gaseous molecules, such as carbon dioxide. The results were published in the October 13th issue of the online version of Nature.
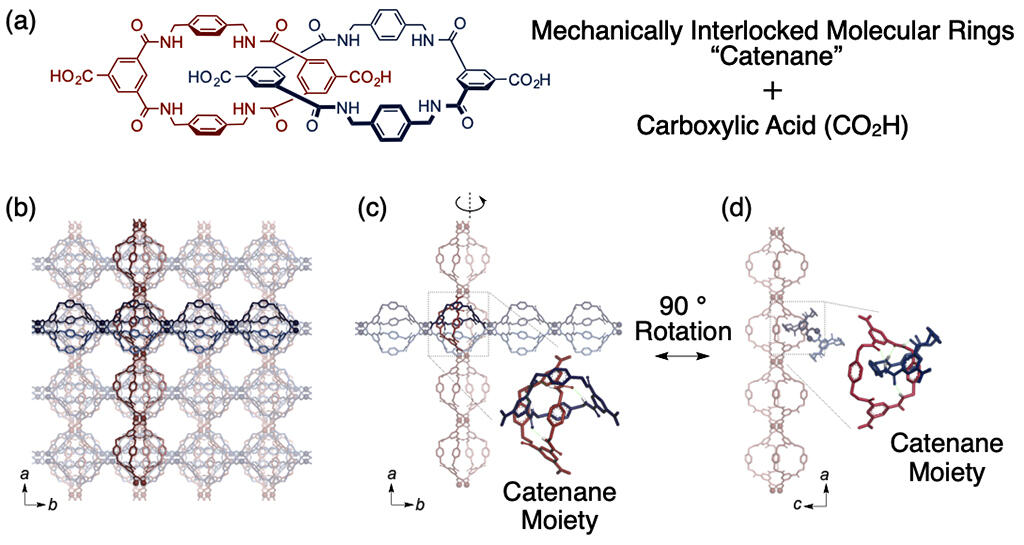
Although there is no direct bond between the constituents, the 3D entanglement formed by the spatial interpenetration of multiple cyclic or rod-shaped molecules is called a topological bond. Unlike ordinary chemical bonds, topological bonds permit molecules to move relatively freely. Catenanes are interlocked structures in which ring-shaped molecules are connected in a way similar to a chain by topological bonds. Therefore, the ring-like molecules that make up catenanes are expected to have a degree of freedom of motion, and polycatenanes, in which large numbers of ring-shaped molecules are linked, are expected to possess unique mechanical properties. On the other hand, a large number of materials developed to date have been reported to randomly contain catenanes, and it was reported in 1995 that crystals composed solely of catenane are clogged with molecules and have no degrees of freedom.
In the present study, the research group examined the feasibility of developing a material that exhibits the mechanical properties that are characteristic of topological coupling by connecting a large number of catenanes and arranging them precisely in three dimensions. Initially, as a method of arranging catenanes regularly and precisely in three dimensions, the group adopted a method of synthesizing crystalline materials from metal-organic frameworks (MOFs). In this method, crystals are formed by the self-assembly of metal ions and ligands.
After this, carboxy groups were introduced at four locations on the catenane molecule, and the cobalt ions that bonded with them were heated in a solvent. The product consisted of green crystals with a width of approximately 0.1 mm and a length of approximately 1 mm. Results of single-crystal X-ray diffraction analyses indicated that that catenane accounts for 90% of the crystal weight, the carboxy groups of catenane are linked by Co2+ to form a linear chain structure, and the chains are linked vertically and horizontally by the mechanical coupling of catenanes. The crystal shrank when the solvent present in the crystal was removed, and its size was restored when it was re-immersed in the solvent.
(Provided by RIKEN with modification from original Nature article)
Single-crystal X-ray diffraction analysis was performed while changing the temperature of the crystal. Results indicate that the structure was flexibly changed even at temperatures of 0 °C and lower. Furthermore, when Young's modulus was investigated by nanoindentation to evaluate the extent of deformation of the material upon the application of a force, the material exhibited the lowest Young's modulus reported to date (1.8 GPa in solvent) among all MOFs and was found to have extreme deformability. After the deformation, the structure was able to be completely restored, even though the material was crystalline.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




