A research group led by Associate Professor Kei Miyamoto of the Faculty of Biology-oriented Science and Technology, Kindai University, and Postdoctoral Fellow Christopher Penfold of the University of Cambridge, has developed a new method for inducing reprogramming in mammalian genomes. By modifying cloning technologies, the researchers succeeded for the first time in the world in inducing the activation of genes specifically expressed in early-stage embryos from the genome of the Scimitar oryx, which is extinct in the wild. In previous studies, the method for analyzing the genome of wildlife was to examine the base sequence of DNA. However, this new technique will allow researchers to analyze how genomic sequences function in a variety of animal species, including extinct animals. These findings were published in iScience.
Provided by Kindai University, Licensed under CC BY 4.0. https://doi.org/10.1016/j.isci.2021.103290
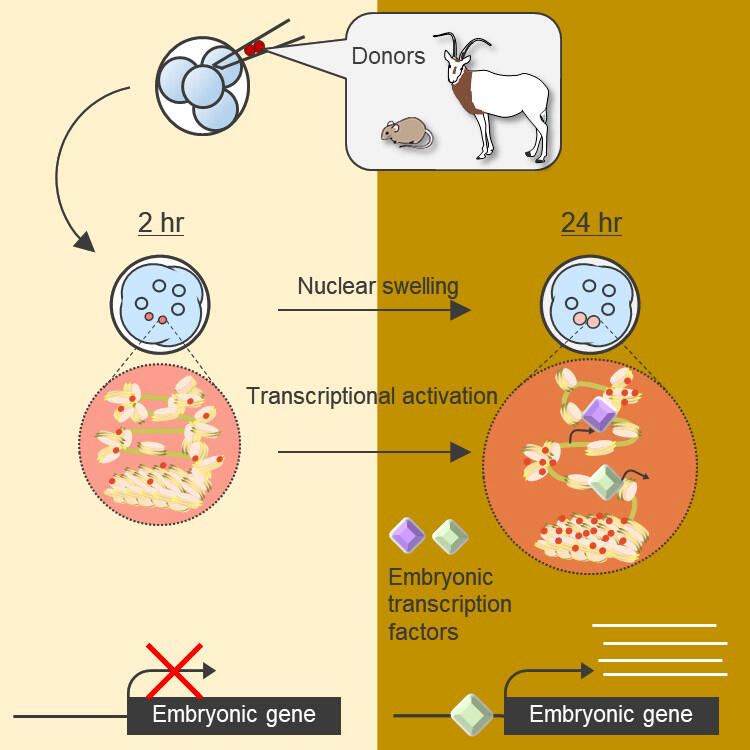
For somatic cell nuclear transfer used to produce cloned animals, a donor cell is needed with the genome of the animal intended to be cloned and a recipient egg of the species into which it will be transplanted. In other words, if wild animal cells are to be reprogrammed using cloning technology, it is necessary to recover their eggs, which is not practical. Therefore, a xenogeneic nuclear transfer method was developed, in which cell nuclei were transplanted into eggs of different species from the donor. In most cases, interspecies nuclear transplantation failed to achieve the activation of embryonic genes, preventing interspecies cloned embryos from dividing normally. Overcoming this issue is essential for the development of cloned embryos.
In an effort to overcome the major barriers of embryonic division abnormalities and embryonic gene activation failures observed when using conventional nuclear transfer methods, the research group aimed to develop a novel nuclear transfer method that could directly induce activation of embryonic genes without requiring the induction of cell division. In conventional nuclear transplantation methods, the cell cycle of donor cells is synchronized with that of the early embryonic recipient cell through timed activation of the embryonic genes, and the genes are activated only after cell division occurs. The researchers focused on this phenomenon and proposed a new nuclear transfer method in which donor cell nuclei are directly transplanted into early embryos that already have transcriptional activity.
Using this novel nuclear transfer method, the researchers first transplanted donor cells from early embryos and allogeneic mice, which confirmed the activation of embryonic genes that were not originally expressed in the transplanted cells. Furthermore, the researchers experimentally showed that activation of these embryonic genes is achievable without cell division or DNA replication. This is the first time direct gene activation has been induced using early mammalian embryos without division and DNA replication. In addition, the group successfully identified transcription factors present in early embryos that are important for embryonic gene activation.
Primary cell cultures were established from tissues recovered from captive scimitar oryxes, which are now extinct in the wild. Using the nuclei of these cells, the researchers investigated whether embryonic gene transfer from heterologous cells is possible using the novel nuclear transfer method. As a result, even when cell nuclei collected from postmortem scimitar oryxes were transplanted, reprogramming was confirmed as in the case of mouse cell nuclei. Activation of embryonic genes was also observed.
The researchers constructed an experimental system to induce transcription from the genomes of mammalian species, including postmortem of wildlife and endangered animals. This new approach allows researchers to obtain information about how genome sequences function, as well as facilitating sequencing of genomes. Genomic sequence function and genome sequencing are very important for understanding biodiversity. Furthermore, this method succeeded in overcoming the abnormal division of embryos and the failure of embryonic gene activation, which have been barriers to the reprogramming of wild animal cells. In the future, the group will further develop this method, take on the challenge of amplifying reprogrammed wild animal genomes, and thereby aim to conserve valuable genomic resources.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




