The auditory nerve is not known to regenerate in adult mammals; however, a research group at Kyushu University discovered that a small number of new neurons emerged in the inner ear of injured adult mice. They also succeeded in significantly increasing the number of neurons by co-administration of growth factor and valproic acid, and as a result, they found that hearing that was previously lost could be restored. The research group was composed of Kyushu University Faculty of Medical Sciences Graduate Student Takahiro Wakizono (at the time of research), Research Fellow Tetsuro Yasui, Assistant Professor Hideyuki Nakashima, Professor Kinichi Nakashima, and others, and had their results published in JCI insight. Professor Nakashima said, "In this study, we have only seen the phenomenon up to 35 days after the procedure. There are many issues to be tackled, such as long-term effects and elucidation of the mechanism by which Schwann cells transform into neurons. We would like to continue basic research to examine the therapeutic effects using large animals and non-human primates and explore the clinical potential."
Provided by Kyushu University
According to the World Health Organization, more than 430 million people, about 5% of the world's population, currently suffer from hearing loss, and it is estimated that nearly 2.5 billion people will have hearing loss by 2050. Hearing loss has traditionally been treated only with hearing aids and cochlear implants, and there was no radical cure. However, in clinical practice, if a certain amount of spiral ganglion (SG) cells including spiral nerve cells, which are the primary auditory neurons, remain, they can be treated in combination with a cochlear implant.
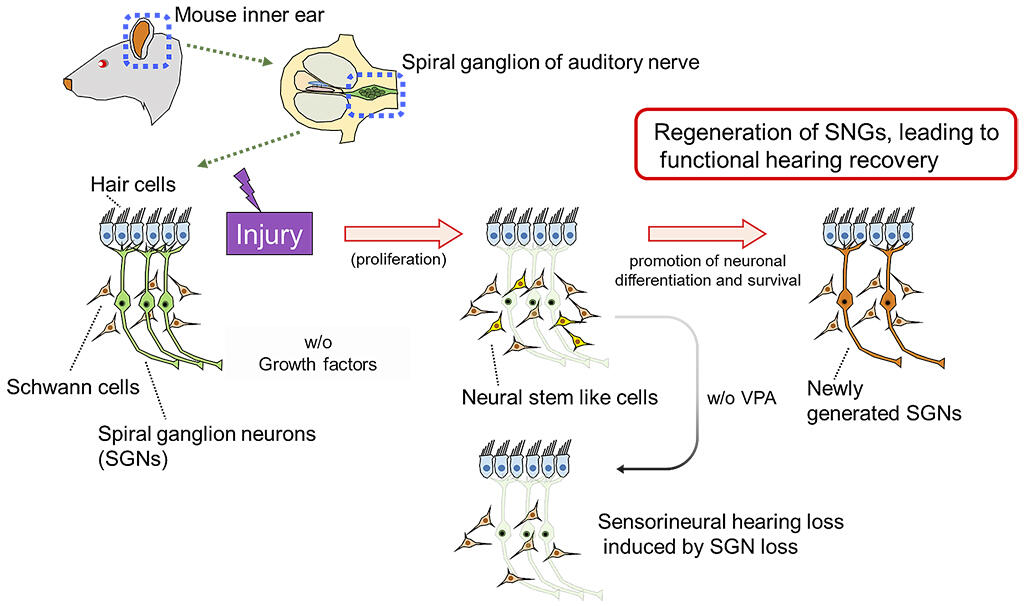
In the SG, it is reported that neural stem cell-like cells appear at the time of injury, but they do not differentiate into neurons; each cell differentiates into glial cells, and no improvement in hearing is observed. By analyzing the injured mouse SG in detail, the research group discovered that some of the proliferative cells that appear at the time of injury are differentiated into neurons (SGN), albeit this a small amount.
Therefore, the research group considered increasing the number of newborn neurons by using growth-promoting factors and differentiation / survival-promoting factors on these proliferative cells that appear after injury to improve the hearing that was lost due to injury. Most cells in the adult mouse SG are composed of dormant SGN and Schwann cells that help the function of neurons, neither of which are proliferative under physiological conditions.
In the developing SG, many proliferative cells are observed on the 14th day of embryonic development. However, cell proliferation is lost around 7 to 14 days after birth, and there are no proliferative cells in the SG of 8-week-old adult mice. Ouabain, which is also used as a cardiac stimulant, has been reported to induce apoptosis of SGN, and when ouabain was administered to the inner ear of adult mice, SGN decreased and proliferative cells appeared. Careful observation revealed a small number of cells that became neurons after proliferation. However, the number of SGN present in the SG was too low to restore hearing after injury.
Therefore, when the growth factor (GF) was administered on the 3rd day after the administration of ouabain, which was when the highest number of proliferative cells could be observed, the number of proliferative cells increased remarkably on the 7th day post administration. However, when observed on the 28th day, the number of SGN cells remained low, and the final SGN number could not be increased by the administration of GF alone.
About 10 years ago, Professor Nakashima discovered that the antiepileptic drug VPA induces differentiation of neural stem cells and enhances neuronal survival by inhibiting histone deacetylase. Therefore, VPA was administered after the administration of GF, and the number of SGN cells was observed 28 days after the injury caused by ouabain.
As a result, it was found that the number of SGN cells was significantly increased by the administration of GF and VPA. Furthermore, using the auditory brainstem response (ABR) test to measure the hearing of mice, when ouabain treatment was performed on normal mice, hearing was completely lost on the 7th day after the treatment. However, in mice treated with GF and VPA, hearing improved 35 days after treatment. This successful neurogenesis in the inner ear, which was thought to be impossible until now, is of great significance. Though currently in the basic research stage, it is expected to lead to the fundamental treatment of deafness including senility in the future.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




