The collaborative research group consisting of Lecturer Madoka Suzuki of the Protein Nanoscience Laboratory of the Institute for Protein Research at Osaka University, Chief Researcher Hiroaki Kubota of the Tokyo Metropolitan Institute of Public Health, Mr. Hiroyuki Ogawa of Waseda University (at the time of the study), Professor Emeritus Shin'ichi Ishiwata of Waseda University, and colleagues discovered that the intracellular mechanism important for the maturation of nerve cells is precisely controlled by temperature.
The fetus of mammals, including humans, grows in the womb of the mother, during which time the axonal process of the nerve cell elongates and connects to another nerve cell. The mechanism of axon elongation involves its tip (growth cone), where elements that exert forces to alter the shape of the cell or regulate the on/off switch of the force are gathered. Typical examples of force-generating proteins are the myosin and actin filaments, and one of the regulators of growth cones is a protein called drebrin E. The concentration of drebrin E has been known to decrease with the maturation of nerve cells, but the details of how this concentration change alters the force were unknown.
In this study, the research group focused on the proteins present in animal fetal nerve cells that are involved in cell shaping by exerting force in the cells as well as the mechanism by which temperature controls the force. They conducted experiments using purified actin, myosin, and drebrin E proteins by combining a local heat pulse method with the experimental microscopy system.
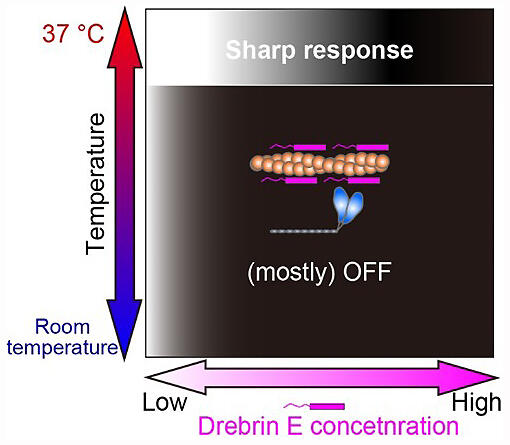
They visualized the interaction between the myosin and actin filaments by using a fluorescent dye to brighten actin filaments with a diameter of less than 10 nanometers. The movement of the actin filaments upon interaction was accurately traced by image analysis. Microscopy observation showed that drebrin E inhibited the interaction between the myosin and actin filaments when the field of view was at ambient temperature.
However, the actin filaments bound to drebrin E started to move as soon as a heat pulse was applied. The researchers repeated the experiment with different temperatures and drebrin E concentrations. The results showed that the on/off status of the force could be altered sharply by slight changes in the concentration of drebrin E, but only when the concentration was within the physiological range and the temperature was at approximately 37°C. Thus, it revealed a feature that can be effectively switched on/off.
Provided by Osaka University
As explained by Dr. Suzuki, "We used a complex of proteins that work on fetal nerves. By finding an answer to the question 'At what temperature does another complex of another organ or organism function? ', we may get closer to the reason why humans have a normal body temperature of approximately 37°C. Furthermore, by understanding the mechanisms based on the commonalities and differences of various complexes, we may be able to create nanoscale machines that alter the functions of those complexes or allow them to work stably depending on the temperature."
■ Local heat pulse method: A method for precisely controlling temperature changes in the field of view in combination with the microscopy technique. Light with a wavelength that is well absorbed by water is harvested by the lens, and a local temperature gradient is instantly formed in the water.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




