The research group composed of Professor Koshi Takenaka, graduate student Yoshifumi Kadowaki, Associate Professor Yoshihiko Okamoto and others of the Graduate School of Engineering, Nagoya University. They announced the discovery of a new material that is eco-friendly and shrinks (negative thermal expansion) when heated. By partially substituting Zn2P2O7 (zinc pyrophosphate) with Mg (magnesium), a continuous negative volume change was realized in the temperature range from −10 °C to 80 °C. Performance comparable to or better than those of conventional negative thermal expansion materials was confirmed for the new material. It is expected to lead to a dramatic improvement in the processing accuracy of electronic devices. The research group has already applied for a patent and will start trial supply to companies in early 2022. The results were published in the November 16 issue of the international scientific journal Applied Physics Letters.
In general, solid materials expand when heated, which has led to problems in the fields of semiconductor device manufacturing and precision equipment manufacturing, which require high accuracy at the nanometer level. To resolve this, a method for controlling thermal expansion by mixing a negative thermal expansion material that shrinks when heated is used. Until now, β-eucryptite (LiAlSiO4), which is inexpensive and has a low environmental load, has been developed and used as a negative thermal expansion material, but its degree of negative thermal expansion is low. Several materials like bismuth-nickel oxide have been developed that enable negative thermal expansion of several to several tens of times larger than those of conventional negative thermal expansion materials by utilizing the volume change accompanying the phase transition. However, all of them were rarely used because they contained expensive elements like Lu and Sc, and Pb, which has a high environmental load.
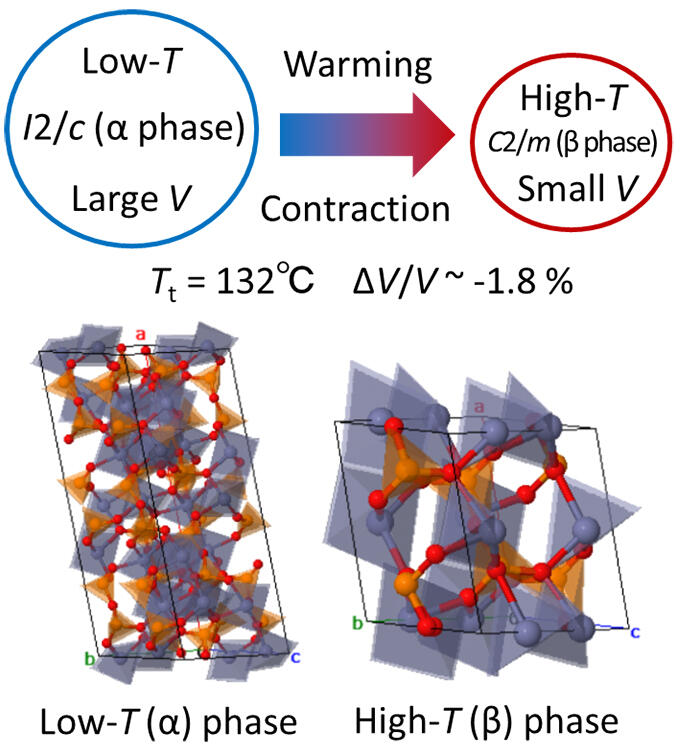
In this study, the research group searched for a new material composed of a wide range of practical, inexpensive, and highly environment-friendly elements, is easy to synthesize, and is low in cost. They eventually focused on zinc pyrophosphate. It was found that zinc pyrophosphate can be easily synthesized by simple mixing of the elements and firing, causing a structural phase transition at 132 °C, and the volume of the high-temperature phase is reduced by 1.8% compared to that of the low-temperature phase. However, sudden volume changes and transition temperatures higher than room temperature created problems for general use.
To combat this, the group focused on magnesium pyrophosphate (Mg2P2O7), which causes a structural phase transition at 68 °C, and aimed at obtaining room-temperature volume change by substituting a part of Zn with Mg. Examining the degree of substitution revealed that the compound in which Zn was replaced with 20% Mg (Zn2-xMgxP2O7) had a large negative thermal expansion, exceeding −60 ppm/°C in terms of the linear expansion coefficient in the temperature range from −10 ° C to 80 ° C including room temperature. The developed negative thermal expansion material is white in color and has been successfully made into fine particles of about 1 μm size. It can be synthesized by a simple operation of firing under normal pressure.
The developed material is an oxide whose main components are abundant and inexpensive elements like zinc, magnesium, and phosphorus, and has high environmental affinity. The degree of negative thermal expansion is equal to or better than that of the giant negative thermal expansion material bismuth-nickel oxide. The developed material was combined with epoxy resin in an experimental setting, and it was confirmed that the large thermal expansion of epoxy resin could be controlled. Controlling thermal expansion by using this material can lead to enhanced functionality of devices and systems, stabilized performance, and extended service life.
Provided by Nagoya University
Currently, the research group is proceeding with technology transfer and plans to establish a venture company. The trial supply from the university to companies can be started early next year. At the same time, the research group plans to proceed with basic research, such as varying the mixing ratio, for product applications. According to Professor Takenaka, "What we expect most is utilization in the electronic device field. The material discovered in this study, which shrinks when heated, controls thermal expansion, and is expected to achieve high functionality, stable performance, and long life in various fields. We believe that this will contribute to the sustainable development of society."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




