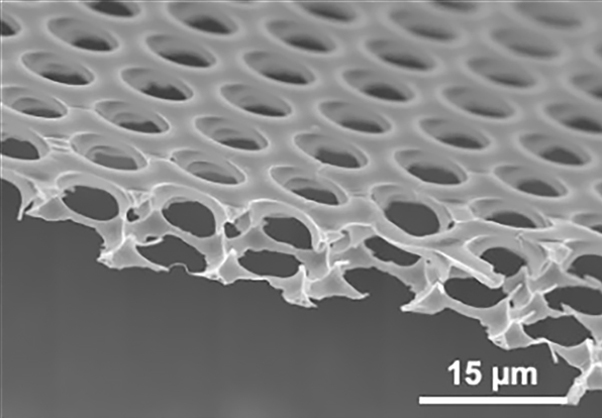
A research group comprising Associate Professor Hiroshi Yabu from the Advanced Institute for Materials Research at Tohoku University, Professor Takehiko Wada from the Institute of Multidisciplinary Research for Advanced Materials at Tohoku University, and others successfully fabricated a membrane that could efficiently separate water and oil, by precisely controlling the wettability of a micron-sized "honeycomb film" with honeycomb-shaped micron-sized pores.
Provided by Tohoku University
Environmental pollution due to oil, such as through heavy oil spills caused by ship accidents, has emerged as a concern. In particular, from the presence of oil in wastewater is one of the important aspects to consider when securing safe water in developing countries. The research group succeeded in adjusting the wettability of the honeycomb film by changing the UV-ozone treatment time; by varying the treatment time, they could fabricate surfaces that were easy or difficult to wet with water. The wettability of the surface was evaluated by measuring the contact angle of water droplets and oil droplets, and it was revealed that the contact angle of the water droplets decreased with the UV-ozone treatment time; water penetrated the pores after 30 min, and oils with low surface tension wetted the honeycomb film.
From this, the honeycomb film with controlled wettability was reinforced with a PET mesh to ensure sufficient mechanical strength; consequently, the film was converted into an oil-water separation membrane. On a surface that is easily wetted with water, the pores of the honeycomb film are always wet when water is first added, and this layer of water prevents oil from permeating the surface, thereby allowing the water to be selectively separated. In contrast, for a surface that is easily wetted with oil, it was found that the oil was selectively separated by pouring the oil first.
The fabricated oil-water separation membrane could efficiently separate a large amount of oil and water from oil-water mixed wastewater, although the membrane thickness was only approximately 1/10th that of hair. In the demonstration experiment, several tens of milliliters of water and oil could be separated using this oil-water separation membrane that was approximately 2 cm in diameter. Since the membrane was not damaged even after separation and the pore size was on the micron scale, it is expected that even small, invisible oil droplets can be separated. Associate Professor Yabu said, "This membrane is expected to be able to separate even small invisible oil droplets. Since a large amount of water and oil can be easily separated, it is expected to be applicable to devices that can easily purify water in developing countries. It is also expected to aid in the quick management of marine pollution caused by oil."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




