The research group of Professor Hideki Ohdan of the Graduate School of Biomedical and Health Sciences at Hiroshima University announced on December 27 that they had started a phase 1 clinical trial of "Peripheral hematopoietic stem cell-derived natural killer cell transfer therapy for the prevention of hepatocellular carcinoma recurrence after hepatectomy" for patients at a high risk of recurrence after hepatocellular carcinoma resection at Hiroshima University Hospital. The research group had previously shown that the survival period and rate after hepatectomy for hepatocellular carcinoma are significantly reduced in patients with recurrence within 2 years.
In 2019, to predict and prevent the high risk of its recurrence, they established a method to detect circulating tumor cells in peripheral blood, using a protein specific to hepatocellular carcinoma (glypican-3) as a marker. In 2021, they established a technology that can predict patients at a high risk of hepatocellular carcinoma recurrence with high probability by using a combination of multiple risk factors. They also found that stage 4 NK cells localized to the liver induced cell death in hepatocellular carcinoma. At the same time, they were working to develop a technology to differentiate and induce stage 4 NK cells from hematopoietic stem cells isolated from the peripheral blood of patients.
Provided by Hiroshima University
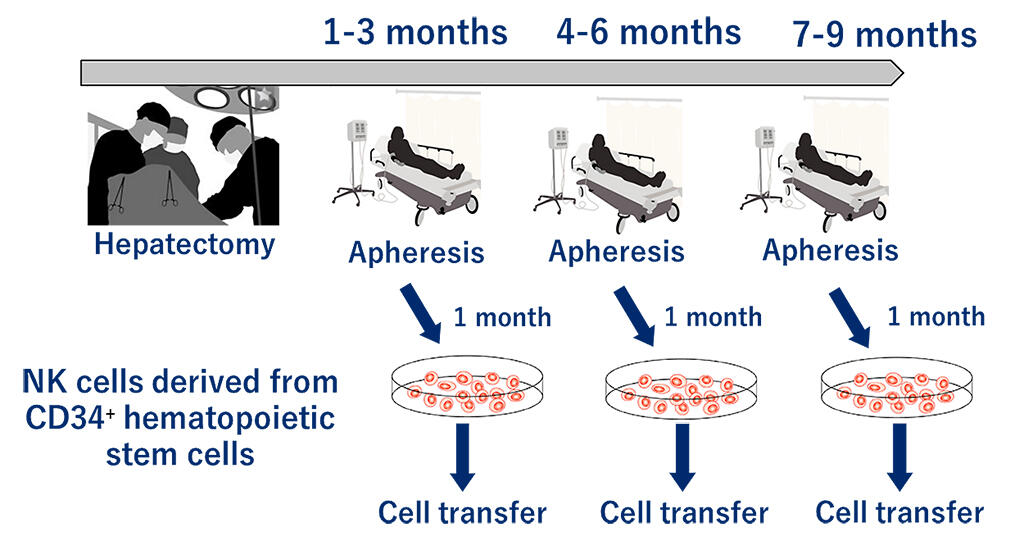
In this phase 1 clinical trial, the research group predicted patients at a risk of recurrence among those who had undergone hepatectomy for hepatocellular carcinoma at the hospital. NK cells derived from the patient's own peripheral hematopoietic stem cells will be differentiated and cultured, which will then be intravenously administered to the patient (postoperative anticancer immunoadjuvant therapy), and the research group will examine the safety and efficacy of this new treatment approach. The administration will be performed at 1, 3, and 6 months after surgery, for a total of three times. Peripheral blood CD34+ hematopoietic stem cells will be collected from patients after hepatectomy, and stage 4 NK cells will be obtained by inducing differentiation after 14-21 days of culture.
It has been approved as a second-class regenerative medicine, and NK cells were administered to the first patient in October. This research group also aims to establish immunomodulatory therapy using iPS cell-derived NK cells in the future.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




