In collaboration with the research groups of Associate Professor Yusuke T. Maeda and Researcher Tatsuya Fukuyama (at the time of research) of the Graduate School of Science at Kyushu University and Professor Takayuki Uchihashi of Nagoya University/Exploratory Research Center on Life and Living Systems of NINS, the research group of graduate student Kosuke Kikuchi, Professor Takafumi Ueno, and Assistant Professor Tadaomi Furuta of the School of Life Science and Technology at Tokyo Institute of Technology, successfully determined the assembly of desired protein architectures.
Proteins are expected to be next-generation nanomaterials derived from living organisms. However, their free design has been challenging because of their complicated assembly mechanism to create patterns.
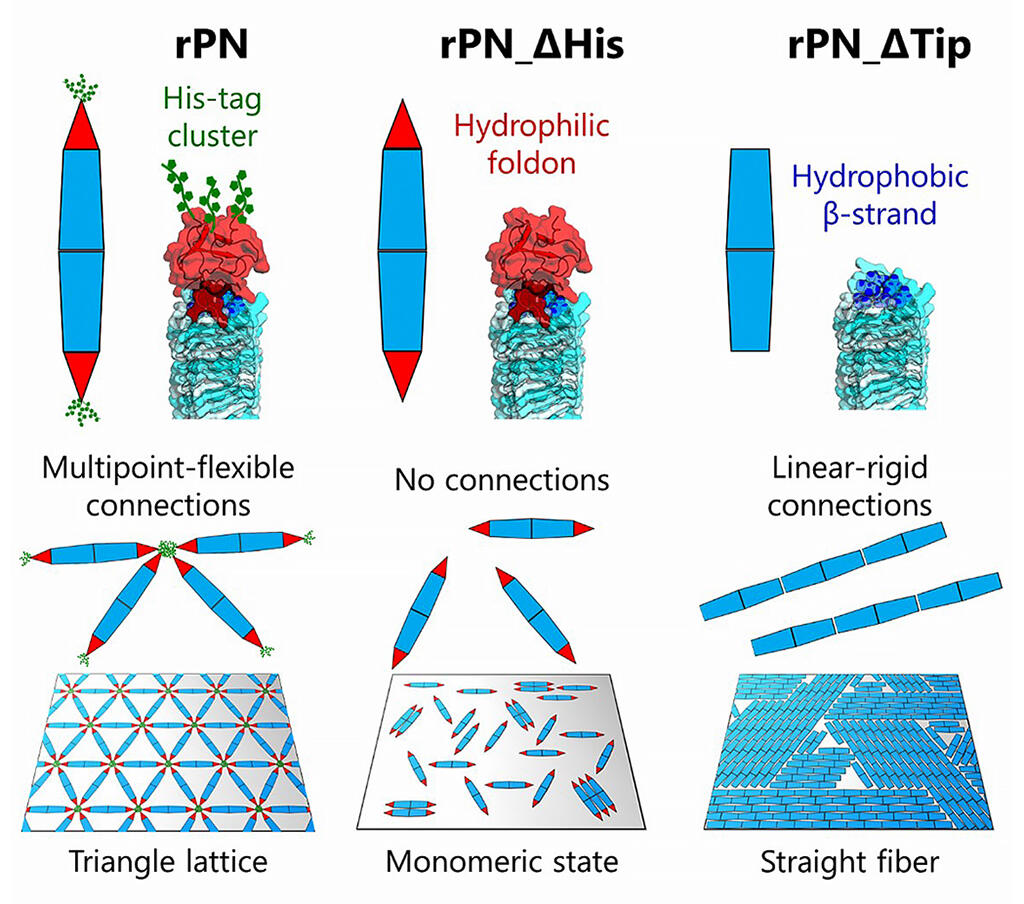
In a previous study on Protein Needles (PN), which are needle-shaped proteins with a rigid body part, the research group found that both ends of the PN can be freely modified. They hypothesized that various two-dimensional patterns could be constructed by designing the bonds between the two ends.
Specifically, they designed three types of PNs: rPN with His-tag clusters extending to both ends, rPN_ΔHis that could no longer form a bond owing to both ends being covered with foldon domains, and rPN_ΔTip with smooth ends owing to hydrophobic β-sheets.
Provided by Tokyo Institute of Technology
Kosuke Kikuchi, Tatsuya Fukuyama, Takayuki Uchihashi, Tadaomi Furuta, Yusuke T. Maeda, Takafumi Ueno: Protein Needles Designed to Self-Assemble through Needle Tip Engineering, Small, 2106401 (2022)
Next, observation of these three types of designed PNs under a high-speed atomic force microscope revealed the formation of a triangular nanolattice, monomeric, and fiber structure, respectively, showing that the two-dimensional nanopatterns could be controlled by the structural design of the PN ends.
Furthermore, the research group was able to directly observe how the rPN with one bonded end searched for and aligned with the bonding partner while rotating in a pivot-like manner. In addition, how the PN assembled in translation and rotation was captured, revealing the dynamic movement of the protein in the formation of the nanopatterns.
The research group constructed a theoretical model based on the mobility observed in these experiments and performed a simulation based on the Monte Carlo method. The result showed a dramatic change in the final nanopattern depending on the difference at the ends of the PN, which was in good agreement with the experimental results.
Professor Ueno commented, "If we can utilize the alignment mechanism clarified by both the observation experiments and theoretical simulations in this study, we can expect to create a molecular group robot that treats proteins like a flock of birds or school of fish. Moreover, we can expect the development of smart materials with unexpected new structures and functionality by enhancing and mixing the protein parts to be assembled."
■ Smart material: A functional material that perceives and judges the surrounding environment and stimuli and then takes appropriate actions based on these.
■ His-tag cluster: A series of six histidines, one of the 20 amino acids, is called a His-tag. In rPN, three His-tags are assembled at each end, resulting in a total of 18 histidines, which is called a His-tag cluster.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




