Some amyloid protein aggregates, which are believed to be the cause of neurodegenerative diseases such as Alzheimer's disease, are highly toxic, while others are weakly toxic. This difference in toxicity was found to be due to the difference in the magnitude of motion of approximately one hydrogen atom.
Senior Researcher Tatsuhito Matsuo of the National Institutes for Quantum Science and Technology (QST), Dr. Alessio De Francesco of Institut Laue-Langevin in France, Professor Judith Peters of Université Grenoble Alpes in France, and their colleagues jointly revealed for the first time that the magnitude and speed of atomic motion differs among amyloid protein aggregates with different cytotoxicity profiles. Senior Researcher Matsuo commented, "For treating Alzheimer's disease, drugs targeting neurotransmitters are available, and drugs for suppressing aggregate formation are being developed. With our discovery of the atomic motion that controls the intensity of cytotoxicity, approaching the aggregates themselves may lead to the development of drugs that delay or stop the progression of the disease." Their findings were published in Frontiers in Molecular Biosciences.
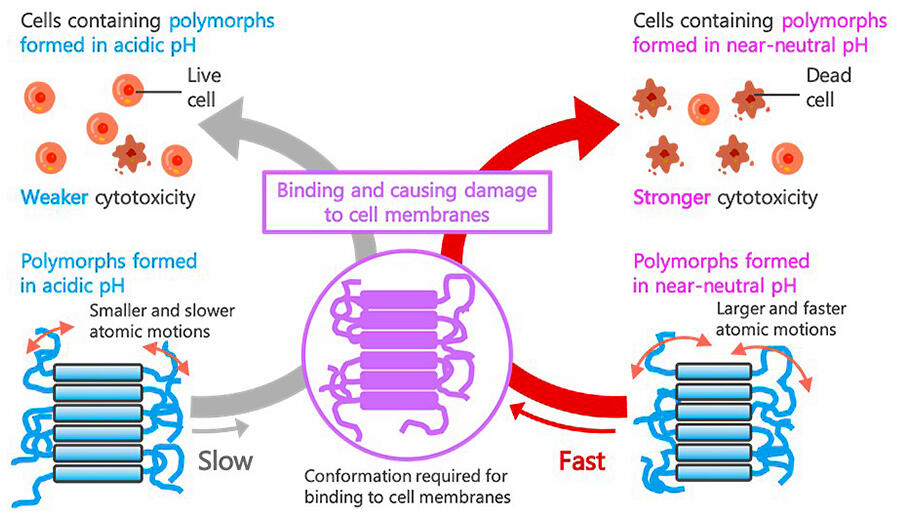
Alzheimer's disease (AD) is a common neurodegenerative disease that affects 1 in 200 individuals, or 1 individual every 7 seconds, worldwide, and poses major economic burden on families and society. Destruction of surrounding cells by amyloid protein aggregates is believed to cause this disease. In addition, with regards to the amyloid model, it has been reported that aggregate A, composed of dispersed fibers wherein lysozyme is aggregated under acidic conditions, exhibits weak cytotoxicity, while aggregate N, composed of aggregated short fibers wherein lysozyme is aggregated under neutral conditions, exhibits strong cytotoxicity. Although the hypothesis that "the motion of highly toxic aggregates is more intense than that of weakly toxic aggregates" has been proposed to explain the differences in their cytotoxicity, it has not been confirmed so far.
To test this hypothesis, the research group analyzed the motion of the aggregates at the atomic level using an inelastic neutron scattering instrument installed in one of the world's largest research facilities at Institut Laue-Langevin. The analysis showed an increased and faster motion of atoms in the highly toxic aggregates than that in the weakly toxic aggregates. The speed of the motion differed by approximately 1.5-fold, and the magnitude of the motion differed by approximately 1 angstrom. This indicates that even a slight difference of the size of approximately one hydrogen atom can determine the properties of the aggregates.
Provided by QST
Aggregates bind to the cell membrane and cause toxicity. The constituent proteins of the aggregates move and change their form, allowing them to easily bind to the cell. Highly toxic aggregates quickly reach the cell membrane and exhibit large motions (changes) to transform into a form that easily binds to the cells and exerts cytotoxicity. The difference in motion on a time scale of hundreds of picoseconds was found to be responsible for the 3-fold difference in cytotoxicity.
Senior Researcher Matsuo said, "We would like to proceed with research that will lead to the development of new therapeutic agents and methods by conducting computational simulations and collaborative research with laboratories capable of conducting animal experiments."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




