The innovative technique is a world's first for Hokkaido University
There are many proteins involved in the spread of cancer. However, some of them are notably difficult to observe in patient tissue samples. Now, to tackle this challenge, a research group from the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) of Hokkaido University has developed an unprecedented technique. Their method allows the use of cancer tissue removed from colorectal cancer patients to evaluate the ability of cancer cells to move on a tissue section.
Rac and Cdc42* are well-studied low molecular weight G proteins that regulate the ability of cancer cells to move, which is called their motility and invasive capacity. The more active these molecules are, the greater the ability of cancer cells to move. This also makes it more likely that diseased cells will invade and metastasize in blood and lymph vessels.
Until now, the motility and invasive capacity of cancer cells had only been assessed biochemically with a method called Rac/Cdc42 pull-down assay. However, when using this approach, the positional information within the cancer tissue is completely lost, and it is also impossible to evaluate the heterogenicity of the cells (how different they are from each other).
Fortunately, and for the first time ever, a research group including Professor Shinya Tanaka and Associate Professor Masumi Tsuda of the Department of Cancer Pathology, Faculty of Medicine, Hokkaido University/Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), managed to successfully evaluate the motility and invasive capacity of cancer cells in cancer tissue sections removed from colorectal cancer patients. The study has been published in Scientific Reports.
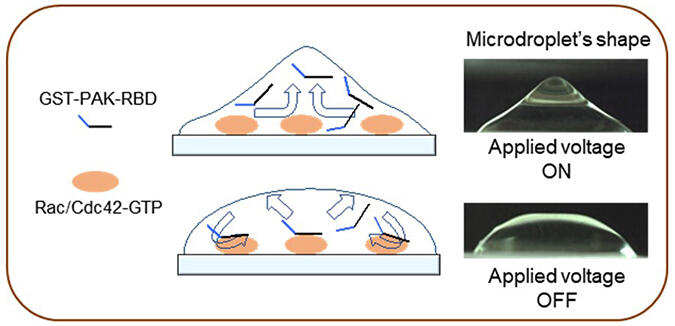
The researchers used a recently developed non-contact agitation technology in which alternating electrical fields are used to 'stir' liquid droplets at extremely high speeds. With this approach, the group found that they were able to rapidly and specifically evaluate the motility and invasive ability of colon cancer cells by agitating a solution containing molecular probes that bind to activated Rac/Cdc42 on cancer tissue sections. Such sections are called formalin-fixed paraffin-embedded (FFPE) specimens and are routinely used for physical diagnosis.
Their evaluations revealed many insights about the Rac/Cdc42 activity of cells. (a) First, that it was significantly higher in the tumor area than in the normal mucosa of the colon. (b) Moreover, the more advanced the cancer cell stage, the greater the increase in Rac/Cdc42 activity. (c) This increase was particularly high at the leading edges of the tumor, where cancer cells infiltrate the surrounding healthy tissue. (d) Additionally, in cases where Rac1/Cdc42 activity was high, there was a strong tendency for lymphatic invasion.
According to Associate Professor Masumi Tsuda, "While developing this technique, we found it difficult at first to suppress the staining background and detect specific signals. This technology is effective for breast cancer and brain tumors as well as colorectal cancer, and it promises to provide useful information for predicting lymph node metastasis and for the assessment of Rac inhibitor-based therapies in the future."
■ Rac/Cdc42: Major members of the Rho family of low molecular weight G proteins. They become active upon binding with GTP (guanosine triphosphate), and are inactivated upon binding with GDP (guanosine diphosphate). The motility and invasive ability of cancer cells are increased by activated Rac/Cdc42.
(Provided by Hokkaido University)
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




