The research group, composed of Professor Alex Hiro Mayo and Professor Shintaro Ishiwata of the Department of Materials Engineering Science at the Osaka University Graduate School of Engineering Science, successfully synthesized high-pressure single and polycrystals of the phosphorus-based Zintl compound MoP4, with a black-phosphorus-derived layered structure. The research group found it to be a promising platform for exploring new topological semimetals.
Provided by Osaka University
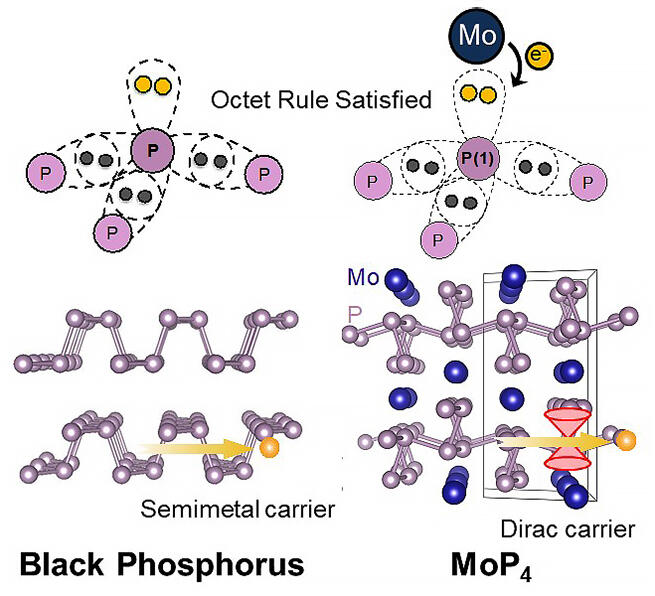
Phosphor is one of the main elements that constitute various functional substances, such as optical communication materials and biological substances such as DNA and phosphoric acid. The various structures and functions of phosphorus compounds are attributed to the octet rule. The layered structure and electronic structure of black phosphorus, which is expected to serve as a post-graphene material or as an ultrafast semiconductor, is also governed by the octet rule.
A Zintl compound is a general term used for compounds consisting of non-metal elements and metal cations that form covalently bonded polyanionic clusters, and the crystal as a whole possesses intermediate characteristics between those of ionic and covalently bonded crystals. This feature is attributed to the polyanion cluster fulfilling the octet rule via lone-pair formation by electron supply from the metal cation, which makes it easy to create a semiconductor or semimetal electronic structure. However, elemental phosphorus is known to form various polyanion clusters under a pressure of tens of thousands to hundreds of thousands of atmospheres, and black phosphorus, one of the high pressure phases, is known to be a Dirac semimetal candidate under pressure.
With this in mind, the research group focused on the Zintl compound MoP4, which has a layered structure similar to that of black phosphorus, as a system that can be applied for rationally designing Dirac semimetals by high-pressure synthesis. This substance also has, without exception, a layered phosphorus skeleton similar to that of black phosphorus, but the addition of molybdenum results in a special electronic structure corresponding to a Dirac semimetal rather than a semiconductor.
Through their research, the research group was able to clarify the possibility of generating and controlling a topological electronic structure on a real-space basis through the hybridization of the M2+ valence electron orbital of the metal cation inserted between the phosphorus polyanion layers and the phosphorus p-orbital. The corresponding electronic structure is expected to have the same properties as those of graphene, and may potentially be utilized in next-generation ultra-high-speed, energy-saving electronic devices. Furthermore, it has greatly expanded the possibilities of phosphorus-based compounds as electronic materials. However, since many conductive compounds with a phosphorus skeleton require an ultrahigh pressure of approximately tens of thousands of atmospheres for synthesis, it is difficult to synthesize a pure single crystal or polycrystal sample. This also indicates that many unknown high-performance phosphorus compounds might occur under high pressure conditions.
This research was published in the December 2021 issue of the Journal of the Physical Society of Japan, which is an English journal published by the Physical Society of Japan. According to Professor Ishiwata, "Practical functional materials require characteristics such as low manufacturing cost, safety, and stability, in addition to superior functionality. In this context, electronic materials containing phosphorus lattice have great potential for practical applications, and we would like to proceed with the high-pressure synthesis of binary phosphorus compounds containing various transition metals and rare earth metals in the future."
■ Octet rule: An empirical rule that compounds and ions exist stably when the number of outermost electrons in an atom is eight.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




