A research group that includes Keiga Fukui, Graduate Student of the Department of Materials Science and Engineering, Tokyo Institute of Technology, Professor Hideo Hosono, Professor Emeritus of the Materials Research Center for Elemental Strategy, Tokyo Institute of Technology, Soshi Iimura, Senior Researcher at the National Institute for Materials Science, Albert Iskandarov, Specially Appointed Associate Professor, Yokohama City University, and Professor Tomofumi Tada of Kyushu University, has created a lanthanum acid hydride (LaH3-2xOx) containing high concentrations of hydride ions (H-), which are hydrogen-anions, with x values below 0.25, and achieved the world's highest ionic conductivity at room temperature.
Hydrogen is the most abundant element in the universe and has two types of ions: protons that are positively charged and hydrides that are negatively charged. As the charge and the atom move simultaneously, conduction of ions can be applied differently from electronics, in which only the movement of electrons is utilized. Fuel cells that use proton transfer are an example that utilize ion conduction. Even though many proton-mobilizing substances have been known, it is only in the last seven years that substances have been found in which hydride ions are mobilized. This is due to their overwhelmingly larger size than protons, making them less likely to move. If hydride ions with these unique properties can be made to move at high speed in a solid, then high ion conductivity can be achieved. Electrochemical devices with entirely new functions can be realized, such as fuel cells using hydrides as raw materials and new high-output storage batteries. However, the hydride ion has the same size (∼1.2 Å) as the oxygen ion, resulting in significantly lower conductivity at room temperature compared to protons, which are smaller, making it an obstacle in material development.
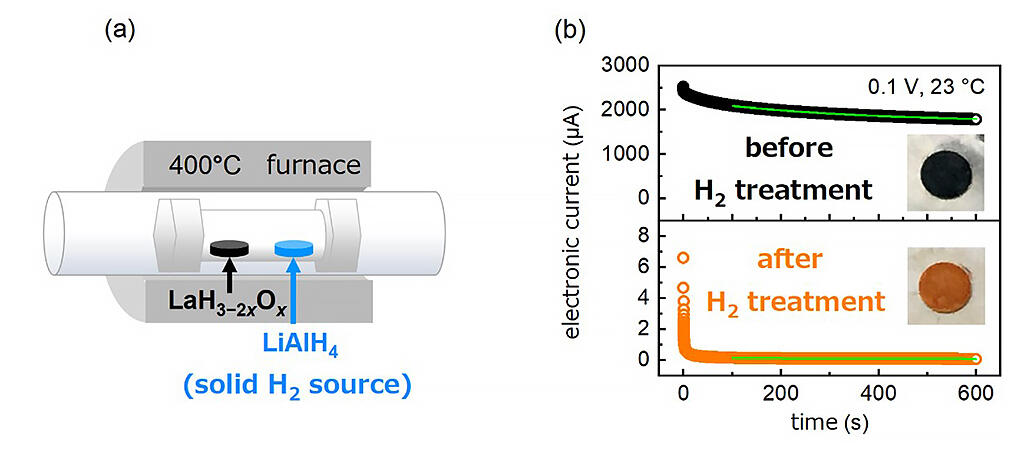
In 2019, the research group used a hydrogenated lanthanum with a higher oxygen content x to achieve a high hydride ion conductivity of more than 10-2 S/cm2 at a medium temperature range of 350 °C. This time, to compensate for the hydrogen deficiency generated in the lanthanum hydride in which the oxygen amount x is suppressed to less than 0.25, after synthesis, a sample was produced by reheating at 400 ° C together with a hydrogen source (a compound that generates hydrogen gas). As a result, the ion conductivity of the sample was nearly equivalent to the proton conductivity of the hydrate system. Hydride ion conductivity of 4 to 5 × 10-7 S per square centimeter was measured, which is the highest reported conductivity at room temperature among hydride conductors. This corresponds to more than a 1000-fold increase compared to the previously reported maximum room temperature hydride ion conductivity. This value is comparable to that of a good proton conductor. It was also possible to visualize by computation the presence of ions that are easy to move and those that are difficult to move.
Provided by Tokyo Institute of Technology and NIMS
Professor Emeritus Hosono said, "Our results have provided us with guidelines to design conductors that have even higher conductivity. Hydride ions are highly active and can decompose carbon dioxide. Thus, they can enable the promotion of chemical reactions by electrical energy, making it possible to transport and control hydrogen. We hope to contribute to the realization of a carbon-free society using hydrogen."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




