The research team, comprising Associate Professor Haruki Watanabe from the University of Tokyo, School of Engineering, Department of Applied Physics, and former Post-Doctoral Researcher Hoi Chun Po from the Massachusetts Institute of Technology (currently Assistant Professor at the Hong Kong University of Science and Technology), theoretically discovered that an electric charge of precisely one-eighth of the elementary charge (e) is generated at the corner of the crystal of sodium chloride.
In recent years, a series of insulators, called topological insulators, have been attracting world-wide attention. There are various types of topological insulators, but a representative one has the property that "the inside of the substance behaves as an insulator, but the surface contains conducting states". The development of devices that utilize this feature is expected in the future.
As research on topological insulators progresses, it has become clear that some "ordinary (non-topological) insulators" may show interesting properties on their surfaces and corners. However, until now, no concrete examples were known, and there were no guidelines on how to find them. In 2020, the research group revealed that the multipole moments of an insulator are related to the fractional charge appearing at the corners of the crystal, and they derived a formula that generally represents the magnitude of this fractional charge. When this formula was applied to various substances, it became clear that the simplest ionic crystal, sodium chloride, is an example of a substance that has a charge quantized to e/8 at the corners of the crystal. Furthermore, the research group performed analytical and numerical calculations based on several models of sodium chloride and confirmed that the abovementioned prediction was correct.
The research group also theoretically examined whether it was possible to experimentally observe the fractional charge appearing at the corner of this crystal, and it was found that it can be observed by measuring the Coulomb force acting between the test charge and angular charge using an atomic force microscope. From these findings, it was concluded that the salt crystal is actually an example of "an insulator having a charge quantized into a fraction of the elementary charge at the corner of the crystal." This would be great news if verified.
According to Associate Professor Watanabe, "For now, it is just a computational result based on theory. It will be great news if the experimental verification of the fractional charge predicted yields a positive result through producing high-purity crystals and performing high-precision measurements. Since we can expect that various ionic crystals other than salts have fractional charges on the surface and corners, we are interested in their effect on the properties of matter, such as the mechanism of dissolution."
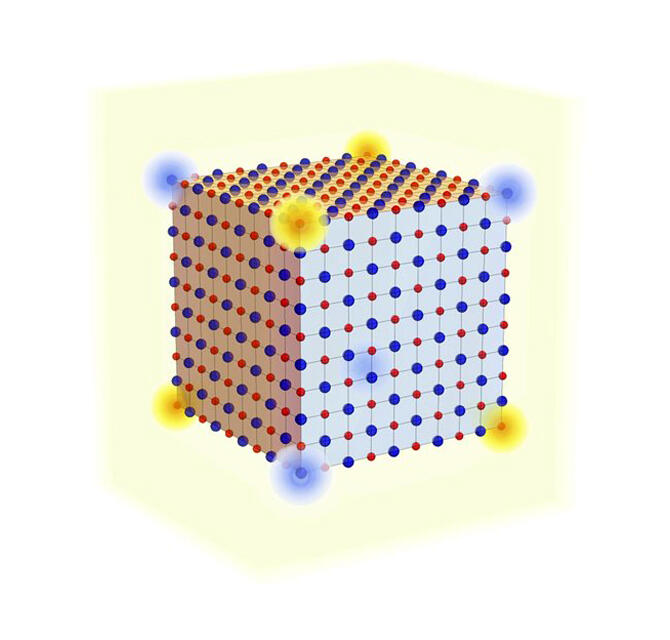
Provided by Associate Professor Haruki Watanabe, The University of Tokyo.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




